Per- and polyfluoroalkyl substances (PFAS) are man-made chemicals used in a wide range of industrial and consumer products due to their water- and oil-resistant properties.1
First produced in the 1940s, PFAS have received global attention over the past decade as a result of their adverse effects on human health. PFAS molecules are often referred to as “forever chemicals” due to their resistance to natural degradation and ability to bioaccumulate in organisms.
These molecules have been detected in air, oceans, surface waters, soils, and even remote Arctic regions.2 On top of this, elevated concentrations of PFAS have been found indoors, demonstrating a serious exposure pathway for humans.3
Nearly 15,000 synthetic PFAS chemicals exist according to CompTox, a chemical database maintained by the U.S. Environmental Protection Agency. Environmental scientists and regulators face a growing challenge due to the emergence of new PFAS and the simultaneous release of next-generation PFAS-like compounds.
Keeping on top of new sources, developing dedicated analytical methods and understanding their toxicological effects is difficult. As a result, most of these substances are currently not subject to regulatory monitoring under current environmental laws, particularly with regards to direct measurement in ambient air.
The added complexity of analysis due to the low concentration of these compounds necessitates highly sensitive analytical detection methods.
Conventional methods used for gas-phase sampling predominantly rely on offline analytical methods. These methods employ passive or active air sampling devices to collect samples onto filters and/or sorbents for subsequent laboratory analysis.4
Such methods are important but do not directly address the issue at its source. Due to the time-intensive nature of the sample analysis, long collection periods hinder the understanding of the sources and dynamics of these pollutants in the air. On top of this, offline methods are usually targeted, detecting only known compounds, limiting their ability to identify new or emerging contaminants.
As such, real-time measurement techniques able to detect a wide range of compounds must be used to gain insight into contaminant release into the atmosphere and understand the sources and transport mechanisms of contaminants.
Direct PFAS Analysis via Chemical Ionization
Chemical ionization (CI) using iodide as a reagent ion is an emerging technique that has shown promise as a method for real-time detection of PFAS in air.4–6
The TOFWERK Vocus Aim Reactor (adduct ionization mechanism) is a versatile instrument that utilizes soft ionization to minimize fragmentation, preserving the parent molecular ion. In conjunction with the TOFWERK time-of-flight mass spectrometer, this approach has unprecedented sensitivity, enabling molecular formulas of the detected ions to be accurately assigned with a time resolution of one second.
The study discussed in this article demonstrates the applicability of the Vocus Aim Reactor as a tool for real-time quantitative PFAS detection in air, bringing this innovative technique to a broader audience beyond the scientific community.
The technique analyzes air directly, without the need for sample collection or pre-separation, unlike traditional methods.
It is essential to have a reliable calibration method for PFAS to obtain quantitative data. Usually, CI can be calibrated using a multi-component gas cylinder for compounds with high enough vapor pressure and limited reactivity.
Alternatively, certified permeation tubes with known permeation rates can be employed; however, these standards are limited.
Less volatile molecules can be prepared into solutions of known concentration and then evaporated using commercially available liquid calibration systems (LCS). However, these devices have many surfaces at which very low volatile molecules can adhere, resulting in long response times and risking permanent contamination from toxic compounds such as PFAS.7
To address these challenges, the study presented here demonstrates a standardized and robust calibration method, including calibration factors, humidity dependencies and detection limits for these emerging contaminants. This method is then also compared with a recently reported calibration approach.3 In addition, two ion chemistries available within the Vocus Aim Reactor were evaluated: iodide and nitrate.
This method is also extended to other emerging contaminants, including pesticides, which present similar problems in gas-phase measurement and calibration due to their high toxicity and contamination issues.
Experimental Details
A Vocus 2R equipped with an Aim Reactor with a measuring frequency of 0.5 Hz was used to perform calibration measurements, the details of which can be found in previous publications.8 The reactor was operated at 50 mbar and 50 °C for both iodide and nitrate ion chemistries.
Liquid calibration standards of PFAS were prepared in methanol, methyl acetate and dichloromethane at concentrations between 0.2 and 4 mg L-1 to reveal any solvent dependency in the calibration outcomes.
A 250 μL glass syringe (Hamilton) and a syringe pump (KD Scientific) were both employed to compare the precision, stability and reproducibility of single and continuous injections of liquid standards, respectively. The injection rates tested ranged from 20 μL per hour to 800 μL per hour.
Single injections involved introducing a specific volume of the liquid standard at one time, while continuous injections we used to help maintain a steady flow of the liquid standard over time. This aim of this comparison was to determine which method provided more consistent and accurate calibration results by assessing the precision, stability, and reproducibility.
The sample was introduced into a 130 °C heated 0.5 inch OD Sulfinert tubing at a 90° angle through a GC septum into the injection point. To allow for easy sonication and tubing replacement, the tubing was encapsulated with a heating element. The injector was continuously flushed with 2 sLPM of UHP N2, with excess flow directed to the exhaust.
The experimental setup used for calibration is shown in Figure 1. Due to the ability of the Aim Reactor to switch between reagent ions, a subset of the chemicals was calibrated in both iodide and nitrate modes.
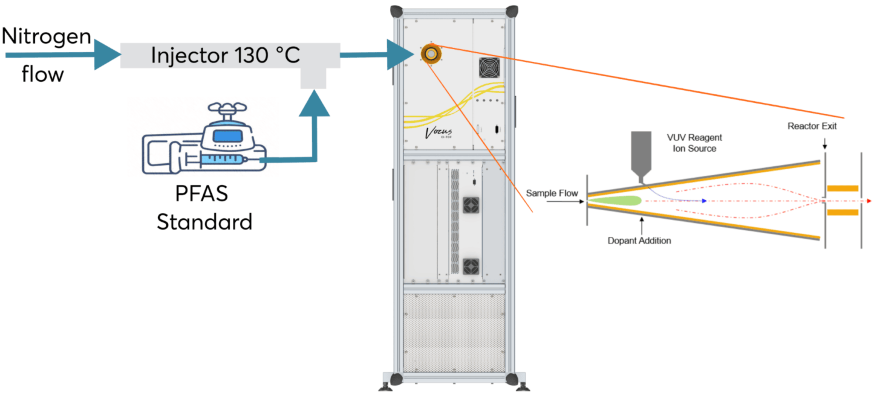
Figure 1. Experimental set up used in the calibration of the PFAS. Image Credit: TOFWERK
Table 1. Iodide CIMS emerging contaminants calibration summary. All calibration factors are reported in counts per second (cps) per part per trillion by volume (pptv) and normalized per million counts of total reagent ion signal. Source: TOFWERK
| Compound |
Calibration factor (ncps ppt-1) |
LOD 1s (ppt) |
LOD 1min (ppt) |
LOD 1min (ng/m3) |
| TFA |
4.30 |
30.0 |
4.0 |
18 |
| 6:2 FTOH |
5.40 |
1.6 |
0.3 |
6 |
| 8:2 FTOH |
5.50 |
1.5 |
0.2 |
4 |
| PFBA |
5.29 |
1.3 |
0.2 |
2 |
| PFPeA |
5.92 |
1.7 |
0.2 |
2 |
| PFHxA |
5.27 |
0.9 |
0.1 |
1 |
| PFHpA |
4.29 |
1.0 |
0.2 |
3 |
| PFOA |
2.77 |
1.3 |
0.3 |
6 |
| PFNA |
1.86 |
2.0 |
0.3 |
6 |
| PFDA |
0.77 |
3.0 |
0.5 |
11 |
| PFUnA |
0.36 |
3.4 |
0.5 |
13 |
| PFDoDA |
0.16 |
4.7 |
0.7 |
19 |
| PFTriDA |
0.06 |
7.6 |
1.2 |
36 |
| PFTeDA |
0.03 |
6.5 |
1.0 |
32 |
| DDT |
0.29 |
5.0 |
0.7 |
10 |
| Pentachlorophenol |
0.29 |
14.0 |
2.0 |
22 |
Results
Table 1 displays the calibration factors and limits of detections (LODs) for 2 fluorotelomer alcohols (FTOHs), 11 perfluorinated carboxylic acids (PFCAs), pentachlorophenol and 4, 4 DDT.
To evaluate potential interferences based on the dilution medium of the injected solutions, the LODs were measured in both nitrogen and ambient air. The 1 Hz LODs were within 10 % between the matrices and found to be a few parts per trillion (ppt). The LODs were reduced by almost an order of magnitude by increasing the averaging time to 1 minute.
Figure 2 displays the calibration curves from a single experiment for 8:2 FTOH, PFBA, PFHxA and PFOA employing iodide as the reagent ion and the syringe pump approach. Good linearity (R2 > 0.98) was found for the calibrated PFAS, even at lower ppt levels, demonstrating the excellent ability of the AIM reactor to detect PFAS in the low ppt range.
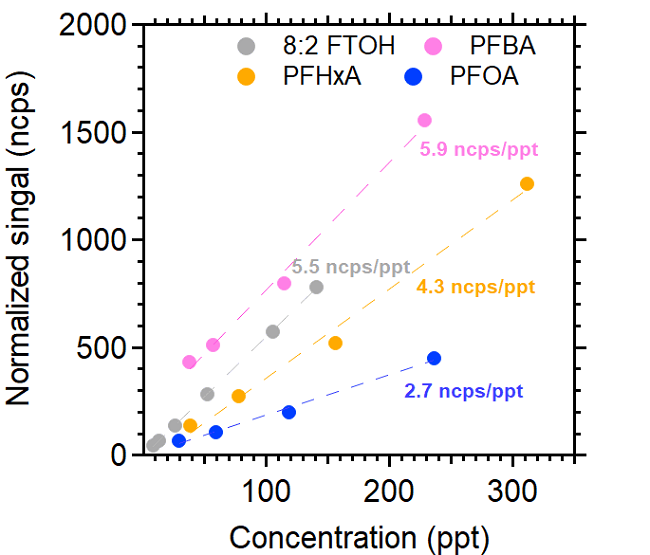
Figure 2. TOFWERK iodide Aim calibration curves for selective PFAS. Image Credit: TOFWERK
The sensitivity of perfluorocarboxylic acids (PFCAs) was also analyzed using NO3 as the reagent ion. On average, the measured sensitivities were within 20 % of those attained with the iodide mode, as shown in Figure 3, demonstrating that the nitrate reagent ion is similarly effective for detecting this subgroup of PFAS.
For both ion chemistries, sensitivity decreased as PFCA molecule size increased, a possible result of their ability to be efficiently transferred to gas phase via this calibration approach.
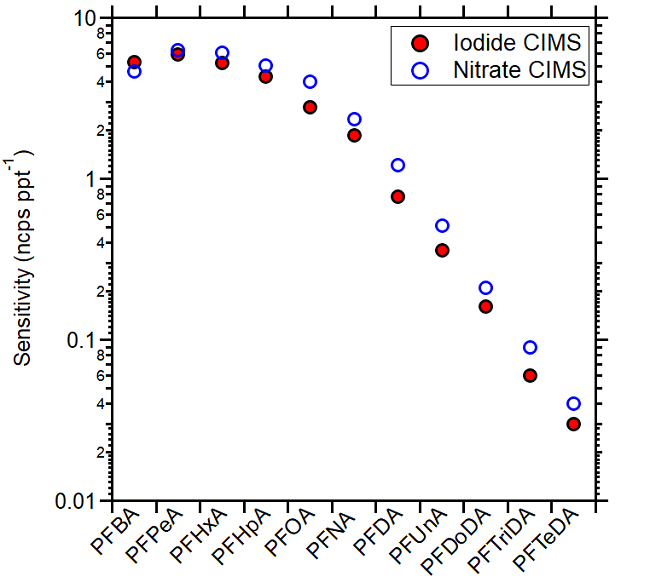
Figure 3. Measured PFCAs sensitivities using iodide CIMS (red full circles) and in nitrate CIMS (blue open circles). Image Credit: TOFWERK
Figure 4 displays the results of the evaluation of the two calibration approaches (single syringe and continuous syringe pump injection). These two methods generally agree within 30 % for the volatile fraction of the PFAS.
However, high variability in the signal response was seen for some molecules with single injections of the same volume and concentration, as shown in Figure 4c.
While the direct injection method is comparatively simple and cost-effective, variability is introduced by human involvement. Contrastingly, the syringe pump method demonstrated enhanced stability in response to changes in injected flow rates and mitigated operator-induced errors effectively. As a result, the remaining results reported here were obtained using the syringe pump method.
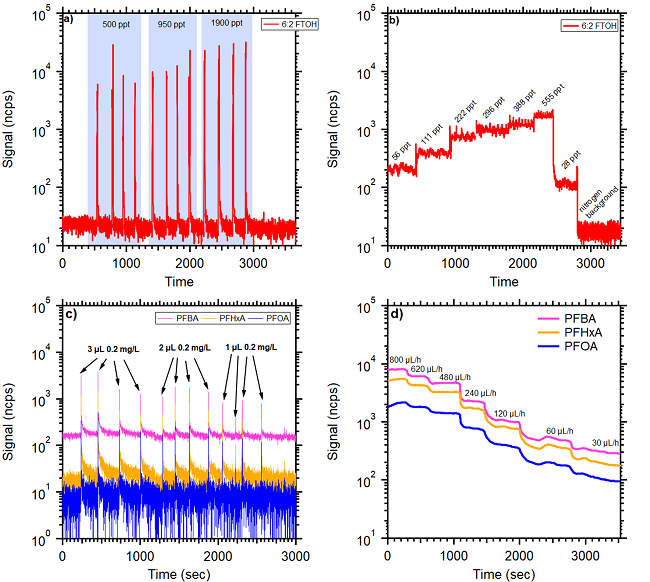
Figure 4. Comparison between direct injections (a & c) and syringe pump approach (b & d) for 6:2 FTOH and selected PFCAs. For manual injections, the same volumes of solution with increasing concentration were used. For syringe pump injection one concentration solution with varying injection rates was used. Calculated mixing ratios of 6:2 FTOH in the air are highlighted by blue shaded area. Image Credit: TOFWERK
Understanding of how sensitivity changes with varying humidity levels is crucial to accurately measure in environments where relative humidity (RH) fluctuates, such as ambient air.
RH-dependent sensitivities vary across compound classes, significantly impacting the sensitivity of species detected by CI at different RH. As a result, consuming calibrations for different RH conditions are required to perform measurements in environments with varying humidity.
The Vocus Aim Reactor utilizes a water vapor control system consisting of a regulated flow of 5 sccm of acetonitrile as a so-called dopant molecule to mitigate the effects of varying humidity.
The dopant displaces water molecules that would usually attach to the reagent ions, meaning the modified reaction mechanism has little dependence on varying water vapor conditions.
The relative sensitivity change for various PFAS as a function of increasing humidity while using dopant is illustrated in Figure 5a.
A tiny decreasing trend is apparent; however, the interpretation of this trend is complicated by the inherent error of the measurement, especially with regard to the reproducibility across replicates. In addition, the variation in sensitivity increases with humidity levels, complicating the interpretation further.
Figure 5b shows the influence of matrix solvent selection on the sensitivity of PFAS detection. A significant deviation with dichloromethane was observed, probably due to its higher volatility. Variances between methanol and ethyl acetate were found to be minimal, the latter being preferable due to its lower toxicity.
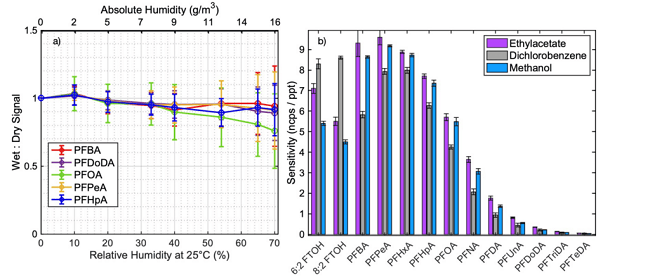
Figure 5. a) Sensitivity normalized to dry conditions as a function of increasing humidity in relative (25°C) and absolute values. The error bars represent the standard deviation, calculated from nine measurements conducted on different days. b) Sensitivity differences for various solvents. Image Credit: TOFWERK
Conclusion
The Vocus Aim Reactor's capability to deliver real-time detection and quantification of PFAS, providing temporal resolution beyond traditional offline techniques, has been rigorously demonstrated in this study.
The technique delivers high sensitivity and specificity, with sensitivities between 0.5 and 5 ncps/ppt and detection limits extending to hundreds of ppq levels. These detection limits exceed typical requirements for background monitoring of ambient air.
However, the precision and speed of the Vocus Aim Reactor lends the technique to real-time identification of volatile PFAS sources and locating possible leaks where concentrations are expected to be significantly higher.
Additionally, the suitability of the Aim for measurements in ambient environments with changing humidity has been demonstrated. The method also has the potential to enhance our understanding of PFAS environmental pathways via indoor air monitoring, atmospheric chamber experiments, consumer products evaluation and material emission testing.
Moreover, the Aim can enable regulatory monitoring of flue gases, ensuring compliance with emission standards.
Acknowledgments
Produced from materials originally authored by Spiro Jorga and Veronika Pospisilova from TOFWERK.
References and Further Reading
- Glüge, J., et al. (2020). An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts, 22, pp.2345–2373. https://doi.org/10.1039/D0EM00291G
- Evich, M. G., et al. (2022). Per- and polyfluoroalkyl substances in the environment. Science, 375, eabg9065. https://doi.org/10.1126/science.abg9065
- Davern, M. J., et al. (2024). External liquid calibration method for iodide chemical ionization mass spectrometry enables quantification of gas-phase per- and polyfluoroalkyl substances (PFAS) dynamics in indoor air. The Analyst, 10.1039.D4AN00100A. https://doi.org/10.1039/D4AN00100A
- Barber, J. L., et al. (2007). Analysis of per- and polyfluorinated alkyl substances in air samples from Northwest Europe. J. Environ. Monit., 9, pp.530–541. https://doi.org/10.1039/B701417A
- Riedel, T. P., et al. (2019). Gas-Phase Detection of Fluorotelomer Alcohols and Other Oxygenated Per- and Polyfluoroalkyl Substances by Chemical Ionization Mass Spectrometry. Environ. Sci. Technol. Lett., 6, pp.289–293. https://doi.org/10.1021/acs.estlett.9b00196
- Bowers, et al. (2023). Evaluation of iodide chemical ionization mass spectrometry for gas and aerosol-phase per- and polyfluoroalkyl substances (PFAS) analysis. Environ. Sci. Process. Impacts, 25, pp.277–287. https://doi.org/10.1039/D2EM00275B
- Mattila, J. M., et al. (2023). Tubing material considerably affects measurement delays of gas-phase oxygenated per- and polyfluoroalkyl substances. Journal Of The Air & Waste Management Association, 73(5), pp.335–344. https://doi.org/10.1080/10962247.2023.2174612
- Riva, M., et al. (2024). Evaluation of a reduced pressure chemical ion reactor utilizing adduct ionization for the detection of gaseous organic and inorganic species. EGUsphere. https://doi.org/10.5194/egusphere-2024-945

This information has been sourced, reviewed and adapted from materials provided by TOFWERK.
For more information on this source, please visit TOFWERK.