Corrosive and toxic chemicals, including tetrabutylammonium hydroxide and p-toluenesulfonyl isocyanate, are employed for the Hydroxyl Number analysis of polyols by titration according to ASTM D4274-16.
This article demonstrates how the XDS RapidLiquid Analyzer operating in the visible and near-infrared spectral region (Vis-NIR) provides a cost-efficient and fast solution for the determination of the hydroxyl (OH) number of polyols without such toxic materials.
With no sample preparation or chemicals needed, Vis-NIR spectroscopy allows for the analysis of polyols in less than a minute.
Methods

Figure 1. XDS RapidLiquid Analyzer and a polyol sample present in a 4 mm disposable vial.. Image Credit: Metrohm Middle East FZC
Polyol samples were quantified with the Analyzer in transmission mode across the full wavelength range of 400 to 2500 nm. Reproducible spectrum acquisition was accomplished by employing the integrated temperature control (experiment carried out at 30 °C) of the Analyzer.
Disposable vials with a path length of 4 mm were utilized, making the cleaning of the sample vessels unnecessary. The software package Vision Air Complete (Metrohm) was employed for all data acquisition and prediction model development.
Table 1. Hardware and software equipment overview. Source: Metrohm Middle East FZC
| Equipment |
Metrohm number |
| XDS RapidLiquid Analyzer |
2.921.1410 |
| Disposable vials, 4 mm diameter, transmission |
6.7402.010 |
| Vision Air 2.0 Complete |
6.6072.208 |
Result
The Vis-NIR spectra, shown in Figure 2, were utilized to form prediction models for quantifying the hydroxyl number in polyol samples.
The quality of the prediction models was assessed using correlation diagrams, which show the relationship between the Vis-NIR calculation and primary method values. The corresponding figures of merit display the prediction’s expected precision during routine analysis (Figure 3).
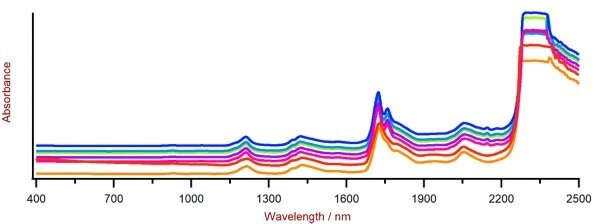
Figure 2. The selection of polyol Vis-NIR spectra was obtained using an XDS RapidLiquid Analyzer and 4 mm disposable vials. For display reasons, a spectra offset was applied. Image Credit: Metrohm Middle East FZC
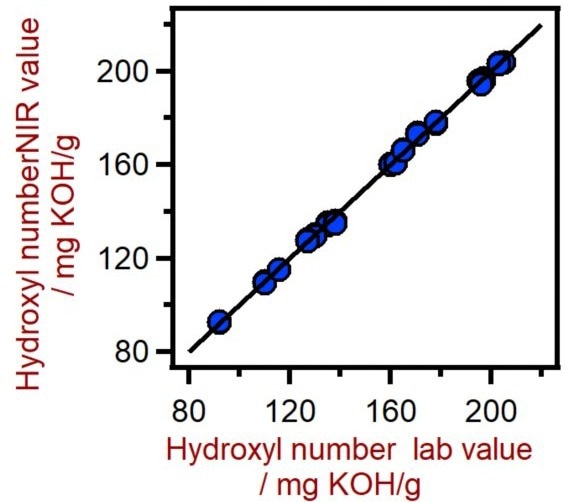
Figure 3. Correlation diagram for the prediction of the hydroxyl number in polyols using an XDS RapidLiquid Analyzer. The Hydroxyl Number lab value was evaluated using titration. Image Credit: Metrohm Middle East FZC
Table 2. Figures of merit for the prediction of the hydroxyl number in polyols using an XDS RapidLiquid Analyzer. Source: Metrohm Middle East FZC
| Figures of merit |
Value |
| R2 |
0.998 |
| Standard error of calibration |
1.28 mg KOH/g |
| Standard error of cross-validation |
1.42 mg KOH/g |
Conclusion
This article demonstrates the feasibility of using NIR spectroscopy for the analysis of the Hydroxyl Number in polyols, as outlined in ASTM D6342-12.
Compared to traditional wet chemical methods, NIR spectroscopy offers significantly lower operating costs (Table 3 and Figure 4). Moreover, unlike ASTM D4274-16, this method eliminates the need for hazardous chemicals during analysis.
Table 3. Comparison of running costs for the determination of the hydroxyl number with titration and NIR spectroscopy. Source: Metrohm Middle East FZC
| |
Lab method |
NIR method |
| Number of analyses (per day) |
10 |
10 |
| Cost of operator (per hour) |
$25 |
$25 |
| Costs of consumables and chemicals OH number |
$6 |
$1 |
| Time spent per analysis |
5 min |
1 min |
| Total running costs (per year) |
$18,188 |
$2,063 |
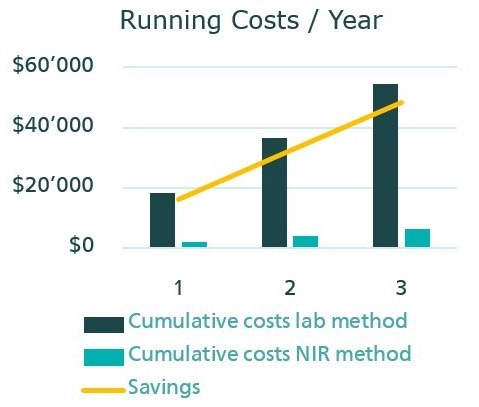
Figure 4. Comparison of the cumulative costs over three years for the determination of the hydroxyl number with titration and NIR spectroscopy. Image Credit: Metrohm Middle East FZC

This information has been sourced, reviewed and adapted from materials provided by Metrohm Middle East FZC.
For more information on this source, please visit Metrohm Middle East FZC.