Soil composition and heavy metal analysis are two closely related topics of environmental research that have gained importance in recent decades. Soil is a critical resource that supports food production and ecological preservation. However, human activities, particularly the release of heavy metals into the environment, are altering its quality.
Heavy metals are naturally occurring elements primarily necessary for organisms in low concentrations but can become hazardous in larger numbers. They infiltrate the soil from various sources, including industrial pollutants, agricultural fertilizers, and wastewater. Heavy metal deposition in soil can seriously affect the environment and human health.
Heavy metal analysis is critical for establishing the amounts of these elements in soil and identifying potential dangers. Modern analytical techniques, such as atomic absorption spectrometry, can accurately detect even tiny amounts of heavy metals. This information is essential for environmentalists, farmers, and governments to take the necessary steps to remediate soil and prevent additional contamination.
This article discusses the quantification of cadmium, chromium, cobalt, copper, lead, manganese, nickel, and zinc in aqua regia extracts under DIN ISO 11047 utilizing the flame HR-CS-AAS contrAA 800 F.
The ISO 11466 standard should be followed when extracting aqua regia from soil, sediment, and sewage sludge samples. The AS-FD autosampler allows for automated sample introduction and dilution.
The high-resolution flame atomic absorption spectrometer contrAA 800 F, which uses a xenon short arc lamp as a continuum source, allows measurement of the absorption of eight metals (cadmium, chromium, cobalt, copper, lead, manganese, nickel, and zinc) in a fast sequential order in one aspiration process.
A 50 mL sample volume can be used to measure 23 absorption lines with high precision in a single step. This specialized AA spectrometer has the unique capacity to analyze the spectrum vicinity of the analyte absorption line to detect spectral overlaps and correct them as needed.
In addition to the distinctive wavelength-independent background correction, the contrAA 800’s unique design eliminates the need to wait for the light source output to stabilize and element-specific slit setups caused by secondary lines.
As a result, the highest possible light throughput is always available, and the element-independent light source is not adversely affected by the analytes’ chemical-physical properties. As a result, the lowest detection limits can be reached by precisely quantifying analytes in even the smallest traces.
The AS-FD autosampler automatically dilutes before measurement and when the highest calibration standard is exceeded. It can also automatically create calibration solutions from a single stock standard and fully automate the standard addition process.
Materials and Methods
Reference Material
- GBW07408 (NCS DC 73326), soil (Institute of Geophysical and Geochemical Exploration, Langfang China)
- GBW07306, river sediment (Institute of Geophysical and Geochemical Exploration, Langfang China)
- BCR-143R sewage sludge with enriched soil (European Commission, Institute for Reference Materials and Measurements)
- BCR-146R sewage sludge of industrial origin (European Commission, Institute for Reference Materials and Measurements)
- BAM-U110 contaminated soil (BAM, Bundesanstalt für Materialforschung und -prüfung, 2006)
- PACS-2 marine sediment (National Research Council of Canada)
Reagents
- Concentrated HNO3 (65 %, p.a.)
- Concentrated HCl (37 %, p.a.)
- Cesium chloride-lanthanum chloride buffer solution (Cs/La) according to Schinkel (10 g L-1 CsCl, 100 g L-1 LaCl3)
- Certified single element standards for Cr, Mn, Co, Ni, Cu, Zn, Cd, and Pb (concentration of the analytes 1,000 mg L-1)
Sample Preparation
The samples were digested using aqua regia in accordance with DIN ISO 11466. The sample material was weighed in 0.3 g aliquots with a filling volume of 50 mL. The sample preparation for flame measurement is according to DIN ISO 11407 standards.
Even lower acid contents for standards and sample dilution than those specified in the norm result in stable measurement solutions.
The samples were diluted for flame measurements with a solution containing 21 % (v/v) concentrated HCl and 7 % (v/v) concentrated HNO3. Chromium and manganese were given an additional 10 % (v/v) of the Cs/La buffer solution.
Calibration
Calibration standards were prepared using 21 % (v/v) HCl and 7% (v/v) HNO3, per the DIN ISO 11047 standard. A solution containing 21 % (v/v) HCl and 7 % (v/v) HNO3 was employed as the calibration blank. Lower acid levels for standards than those specified in the norm also result in stable measurement solutions.
When measuring chromium and manganese with an air-acetylene flame, an additional 10 % (v/v) of the Cs/La buffer solution was used. If these components are determined using a nitrous oxide-acetylene flame, no Cs/La solution is required.
Table 1. Concentrations used for calibration according to DIN ISO 11047. Source: Analytik Jena US
| Standard |
Concentration [mg L-1] |
| Cd |
Cr |
Co |
Cu |
Pb |
Mn |
Ni |
Zn |
| Cal.0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Std. 1 |
0.2 |
1 |
1 |
1 |
1 |
0.4 |
1 |
0.2 |
| Std. 2 |
0.4 |
2 |
2 |
2 |
2 |
1 |
2 |
0.4 |
| Std. 3 |
0.8 |
4 |
4 |
4 |
4 |
2 |
4 |
0.8 |
| Std. 4 |
1.2 |
6 |
6 |
6 |
6 |
4 |
6 |
1.2 |
| Std. 5 |
1.6 |
8 |
8 |
8 |
8 |
6 |
8 |
1.6 |
| Std. 6 |
2.0 |
|
|
|
|
8 |
|
2.0 |
Table 2. Typical calibration functions according to DIN ISO 11047. Source: Analytik Jena US
| Element |
Flame type |
Correlation R2 (adj.) |
Graphic plot |
| Cr |
Air-acetylene |
0.9992
non-linear |
 |
| Cr |
Nitrous oxide-acetylene |
0.9997
non-linear |
 |
| Mn |
Air-acetylene |
0.9993
non-linear |
 |
| Mn |
Nitrous oxide-acetylene |
0.99990
non-linear |
 |
| Co |
Air-acetylene |
0.9993
non-linear |
 |
| Ni |
Air-acetylene |
0.9997
non-linear |
 |
| Cu |
Air-acetylene |
0.9996
non-linear |
 |
| Zn |
Air-acetylene |
0.9994
non-linear |
 |
| Cd |
Air-acetylene |
0.9997
non-linear |
 |
| Pb |
Air-acetylene |
0.9997
non-linear |
 |
| Pb |
Air-acetylene |
0.9990
linear |
 |
Instrument Settings
The contrAA 800 F, a high-resolution flame atomic absorption spectrometer with a continuous emitter as the light source, was used to determine soil extracts under DIN ISO 11466. The 50 mm burner head comes with a scraper.
This ensures automatic cleaning when measuring with a nitrous oxyacetylene flame. The 100 mm burner head is an alternative that improves measurement sensitivity using the air-acetylene flame. To automate the measurement, utilize an AS-F or AS-FD autosampler with a dilution function.
The AS-FD autosampler can automatically perform variable sample dilutions and the conventional addition procedure.
Table 3 shows the instrument characteristics and measurement parameters employed. Table 4 displays the measurement parameters and instrument settings for the method employed. Background correction was performed using iterative baseline correction.
According to DIN ISO 11047, lead’s absorption wavelength is 217 nm. In contrast to the major absorption wavelength at 217 nm, the absorption band at 283 nm is commonly used for lead. The absorption band at 217 nm has a lower signal-to-noise ratio than the line at 283 nm for line sources (such as hollow cathode lamps).
However, with HR-CS-AAS, the main wavelength of 217 nm can be employed without substantial constraints. Both lines were used in this study.
Table 3. General instrument parameters. Source: Analytik Jena US
| Parameter |
Specification |
| Device |
contrAA 800 F |
| Burner type and position |
50 mm, 0° |
| Flame type |
Air/acetylene |
| Measuring time |
5 s, 3 repetition |
| Baseline correction |
IBC |
| Rinsing solution |
1 % (v/v) HCl |
Table 4. Applied method parameters. Source: Analytik Jena US
| Element |
Wavelength
[nm] |
Number of
pixels |
Flame
type |
Gas flow
[L h-1] |
Burner height
[mm] |
| Cr |
357.8687 |
3 |
air/C2H2
N2O/C2H2 |
95
185 |
10
4 |
| Mn |
279.4817 |
3 |
air/C2H2
N2O/C2H2 |
80
180 |
6
4 |
| Co |
240.7254 |
3 |
air/C2H2 |
65 |
6 |
| Ni |
232.0030 |
3 |
air/C2H2 |
45 |
5 |
| Cu |
324.7540 |
3 |
air/C2H2 |
45 |
5 |
| Zn |
213.8570 |
3 |
air/C2H2 |
45 |
4 |
| Cd |
228.8018 |
3 |
air/C2H2 |
45 |
4 |
| Pb |
217.0005
283.3060 |
3 |
air/C2H2 |
60 |
6 |
Results and Discussion
Table 5 shows the typical detection and quantification limitations for the instrument type and measurement conditions. The limits are calculated by the blank value approach, which uses an 11-fold measurement and the 3σ or 9σ standard deviation threshold.
Cadmium, chromium, cobalt, copper, lead, manganese, nickel, and zinc were determined in soil and sediment samples using DIN ISO 11047. Table 6 shows the results of the measurements and compares them to the expected values of the reference materials.
The air-acetylene flame may produce falsely low values for chromium and manganese, even when excessive La is used. Such interferences are almost non-existent in the nitrous oxide-acetylene flame.
To increase the ionization potential of the nitrous oxide flame, extra K or Cs should be added, such as in KCl or CsCl solutions (Cs/K concentration of 0.1-0.2 % [m/m]).
The HR-CS-AA spectrometer contrAA 800 has the unique capability of recording the spectral neighborhood of analytical lines. Spectral overlays can, therefore, be recognized and corrected if necessary. Table 7 displays the relevant spectra for the sample BAM U 110 as an example.
Some analytical lines reveal iron absorption bands in the spectra that do not overlap with the analyte lines. A direct overlap occurs for the zinc analysis line at 213 nm, originating from NO bands. If not adjusted for, this results in an excessively high result.
The LSBC (least-squares background correction) method can be used to adjust overlays so that the analysis line can be detected without interruption. The technique is explained in greater detail below.
Table 5. Achievable limits of detection (LOD) and limits of quantification (LOQ) of the presented method according to the 3σ or 9σ criterion. Source: Analytik Jena US
| Element |
Wavelength
[nm] |
LOD
[mg L-1] |
LOQ
[mg L-1] |
| Cr |
357 |
0.0015 |
0.0045 |
| Mn |
279 |
0.001 |
0.0030 |
| Co |
240 |
0.0016 |
0.0049 |
| Ni |
232 |
0.0028 |
0.0083 |
| Cu |
324 |
0.00073 |
0.0022 |
| Zn |
213 |
0.0015 |
0.0046 |
| Cd |
228 |
0.00067 |
0.0020 |
| Pb |
217
283 |
0.0071
0.0076 |
0.021
0.023 |
Table 6. Measurement results of analyte content determination in soil, sediment, and sewage sludge samples. Source: Analytik Jena US
| Sample |
Element |
Pre-dilution
factor |
Recovery [%] |
Flame type |
Measurement value
[mg kg-1] |
Target value
[mg kg-1] |
NCSDC
73326 |
Cr |
1 |
47 |
C2H2-air |
32.61 |
±0.48 |
68 |
±6 |
| 92 |
C2H2-N2O |
62.31 |
±1.02 |
| Mn |
2 |
101 |
C2H2-air |
659 |
±15 |
650 |
±23 |
| Co |
1 |
97 |
C2H2-air |
12.29 |
±0.087 |
12.7 |
±1.1 |
| Ni |
1 |
100 |
C2H2-air |
31.5 |
±0.24 |
31.5 |
±1.8 |
| Cu |
1 |
92 |
C2H2-air |
22.4 |
±0.13 |
24.3 |
±1.2 |
| Zn |
5 |
98 |
C2H2-air |
66.4 |
±0.9 |
68 |
±4 |
| Cd |
1 |
|
C2H2-air |
<LOQ |
|
0.13 |
±0.02 |
| Pb |
1 |
91 |
C2H2-air |
19.15 |
±0.12 |
21 |
±2 |
| BAM-U110 |
Cr |
1 |
102 |
C2H2-air |
193.5 |
±1.34 |
190 |
±9 |
| Mn |
2 |
101 |
C2H2-air |
585.5 |
±6.6 |
580 |
±19 |
| Co |
1 |
104 |
C2H2-air |
15.07 |
±0.091 |
14.5 |
±0.8 |
| Ni |
1 |
99 |
C2H2-air |
94.9 |
±0.18 |
95.6 |
±4 |
| Cu |
1 |
102 |
C2H2-air |
266 |
±2.4 |
262 |
±9 |
| Zn |
10 |
92 |
C2H2-air |
912.6 |
±5.7 |
990 |
±40 |
| Cd |
1 |
103 |
C2H2-air |
7.24 |
±0.095 |
7 |
±0.4 |
| Pb |
1 |
100 |
C2H2-air |
185.2 |
±0.93 |
185 |
±8 |
| BCR 143R |
Cr |
1 |
98 |
C2H2-air |
417 |
±2.0 |
426 |
±12 |
| Mn |
2 |
98 |
C2H2-air |
840.3 |
±3.4 |
858 |
±11 |
| Co |
1 |
107 |
C2H2-air |
12.67 |
±0.076 |
11.8 |
±1 |
| Ni |
1 |
96 |
C2H2-air |
285.2 |
±7.0 |
296 |
±4 |
| Cu |
1 |
100 |
C2H2-air |
128.8 |
±0.92 |
128 |
±7 |
| Zn |
10 |
94 |
C2H2-air |
999.5 |
±2.4 |
1063 |
±16 |
| Cd |
2 |
98 |
C2H2-air |
70.86 |
±0.53 |
72 |
±1.8 |
| Pb |
1 |
100 |
C2H2-air |
173.7 |
±2.3 |
174 |
±4 |
| BCR 146R |
Cr |
1 |
105 |
C2H2-air |
182.7 |
±3.6 |
174 |
±7 |
| Mn |
2 |
100 |
C2H2-air |
298.6 |
±1.8 |
298 |
±9 |
| Co |
1 |
107 |
C2H2-air |
6.96 |
±0.071 |
6.5 |
±0.4 |
| Ni |
1 |
100 |
C2H2-air |
65.4 |
±0.66 |
65 |
±3 |
| Cu |
1 |
98 |
C2H2-air |
815.2 |
±3.6 |
831 |
±16 |
| Zn |
30 |
95 |
C2H2-air |
2892 |
±50 |
3040 |
±60 |
| Cd |
2 |
100 |
C2H2-air |
18.43 |
±0.17 |
18.4 |
±0.4 |
| Pb |
1 |
101 |
C2H2-air |
588 |
±2.3 |
583 |
±17 |
| PACS 2 |
Cr |
1 |
79 |
C2H2-air |
71.74 |
±0.69 |
90.7 |
±4.6 |
| 99 |
C2H2-N2O |
91.94 |
±0.96 |
| Mn |
2 |
70 |
C2H2-air |
306 |
±3.6 |
440 |
±19 |
| 94 |
C2H2-N2O |
412 |
±7.2 |
| Co |
1 |
89 |
C2H2-air |
10.21 |
±0.05 |
11.5 |
±0.3 |
| Ni |
1 |
90 |
C2H2-air |
35.74 |
±0.39 |
39.5 |
±2.3 |
| Cu |
1 |
101 |
C2H2-air |
310.9 |
±0.59 |
310 |
±12 |
| Zn |
1 |
96 |
C2H2-air |
352.5 |
±6.5 |
364 |
±23 |
| Cd |
1 |
104 |
C2H2-air |
2.2 |
±0.046 |
2.11 |
±0.15 |
| Pb |
1 |
95 |
C2H2-air |
173.1 |
±0.53 |
183 |
±8 |
GBW0
7306 |
Cr |
1 |
50 |
C2H2-air |
94.3 |
±1.6 |
190 |
±24 |
| 89 |
C2H2-N2O |
168,3 |
±4.3 |
| Mn |
2 |
97 |
C2H2-air |
943.8 |
±5.8 |
970 |
±60 |
| Co |
1 |
93 |
C2H2-air |
22.6 |
±0.11 |
24.4 |
±3 |
| Ni |
1 |
89 |
C2H2-air |
69.75 |
±0.40 |
78 |
±7 |
| Cu |
1 |
100 |
C2H2-air |
383.1 |
±3.4 |
383 |
±18 |
| Zn |
2 |
101 |
C2H2-air |
146.3 |
±2.9 |
144 |
±10 |
| Cd |
1 |
|
C2H2-air |
<LOQ |
|
0.43 |
±0.04 |
| Pb |
1 |
92 |
C2H2-air |
24.95 |
±0.081 |
27 |
±5 |
LOQ: Limit of quantification
Table 7. Spectral vicinity of the analysis lines. Source: Analytik Jena US
| Element |
Spectral vicinity |
Remarks |
| Cr (sample BAM U110) |
 |
|
| Mn (sample BAM U110) |
 |
|
| Co (sample BAM U110) |
 |
|
| Ni (sample BAM U110) |
 |
|
| Cu (sample BAM U110) |
 |
|
| Zn (sample BAM U110) |
 |
*LSBC applied |
| Cd (sample BAM U110) |
 |
|
| Pb (sample BAM U110) |
 |
|
| Pb (sample BAM U110) |
 |
*LSBC applied |
LSBC: Least-squares background correction
Correction Spectra
Spectral overlap of the analyte wavelength caused by molecular or other atomic absorption bands can mislead the analysis results. These overlaps can be adjusted via spectral correction. The ASpect CS software can record a suitable corrective spectrum using the LSBC technique to minimize or remove analysis line overlap.
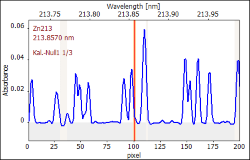
In this series of observations, the LSBC was utilized to measure the NO molecule bands close to the zinc analysis line. The corrective spectrum was generated using the reagent blank value (which had no detectable Zn contamination). Table 8 shows the impact of the LSBC on zinc's analyte absorption line at 213.9 nm.
Table 8. Spectral vicinity of the analytic lines used. Source: Analytik Jena US
| Sample |
Spectral vicinity |
| Blank |
 |
Std. 1
without correction model |
 |
Std. 1
using correction model |
 |
sample BAM U110
without correction model |
 |
sample BAM U110
using correction model |
 |
Summary
The contrAA 800 F provides fast and cost-effective analysis of the metals cadmium, chromium, cobalt, copper, lead, manganese, nickel, and zinc in soil and sewage sludge extracts in compliance with DIN ISO 11047.
The method given in this article outlines the steps required to prepare acid extract samples and analyze them using a flame atomic absorption spectrometer.
The contrAA 800 F’s fast sequential analysis, which allows all relevant analysis lines to be identified in a single aspiration operation without changing lamps, and the AS-FD autosampler’s automated and intelligent dilution, make sample analysis quick and simple.
The analytical performance capabilities of an AA spectrometer are unparalleled and elevate AA analysis to new heights.

Figure 1. ContrAA 800 with AS-FD. Image Credit: Analytik Jena US
Recommended Device Configuration
Table 9. Overview of devices, accessories, and consumables. Source: Analytik Jena US
| Article |
Article number |
Description |
| contrAA 800 F - HR-CS |
815-08000-2 |
HR-CS AAS flame mode |
| AS-FD |
810-60501-0 |
Autosampler for flame analysis with dilution function |
| Burner head 50 mm |
810-60057-0 |
Burner head for the air-acetylene and N2O-acetylene flame |
| Scraper |
812-08000-2 |
Automatic burner head cleaner for the N2O acetylene flame |
References
- DIN ISO 11047:2003-05, Soil quality - Determination of cadmium, chromium, cobalt, copper, lead, manganese, nickel, and zinc in aqua regia extracts of soil - Flame and electrothermal atomic absorption spectrometric methods (ISO 11047:1998)
- DIN ISO 11466:1997-06 Soil quality - Extraction of trace elements soluble in aqua regia (ISO 11466:1995)

This information has been sourced, reviewed, and adapted from materials provided by Analytik Jena US.
For more information on this source, please visit Analytik Jena US.