In this interview, Chady Stephan, Director of Applied Markets at PerkinElmer, discusses how advanced analytical tools are improving battery chemistries, raw material testing, and recycling efficiency to enhance performance and sustainability in the battery industry.
Please can you introduce yourself and your role at PerkinElmer?
I'm Chady Stephan. I manage the applied markets at PerkinElmer, along with a team of end market managers and application scientists.
PerkinElmer is well known for its contributions across various industries. Can you briefly describe how PerkinElmer’s expertise in analytical solutions translates to the battery industry, and what inspired your participation in the Battery Show?
PerkinElmer has been involved in science for more than 85 years. Our portfolio started with molecular spectroscopy and expanded into atomic spectroscopy and chromatography. We've been serving the industrial space for a long time.
With the growth of the battery industry, there’s a growing need for various analytical instruments that ensure batteries are manufactured to specifications and are safe to handle and package. This has driven us to offer solutions and a range of tests specifically for the battery industry.
We're here at the trade show to connect with industry leaders and discuss how our portfolio of analytical instruments can best aid them in advancing battery chemistries, from raw materials to cathodes, separators, and anodes.
PerkinElmer at The Battery Show 2024
Given the importance of material purity and quality in battery performance, how do PerkinElmer's analytical instruments help manufacturers ensure the quality and consistency of key battery materials like lithium, cobalt, and nickel?
We have a long history in the mining industry, and since batteries are made from minerals, there's a connection to raw materials and extraction, where we have a solid understanding of the industry. As lithium batteries emerged, we saw increased demand for analyzing impurities in lithium, especially at the ore stage, when it comes out of the mine.
When raw materials are blended with nickel, cobalt, manganese, and iron to make NMC or LFP batteries, our atomic spectroscopy product lines—primarily ICP-OES—are closely tied to that process, though some industry leaders are now moving toward ICP-MS for high-performance and solid-state batteries. We offer solutions for determining the precise ratio of nickel, manganese, and cobalt when blending NMC cathodes.
As the battery packaging process begins—building the cathode materials, adding the electrolyte, separators, and anodes—our portfolio of instruments is used throughout the assembly process, testing both the batteries and the raw materials and ensuring they meet the quality standards whether you're a supplier or a battery manufacturer.
When you order these chemicals, they need to meet the quality assurance and quality control requirements you're looking for.
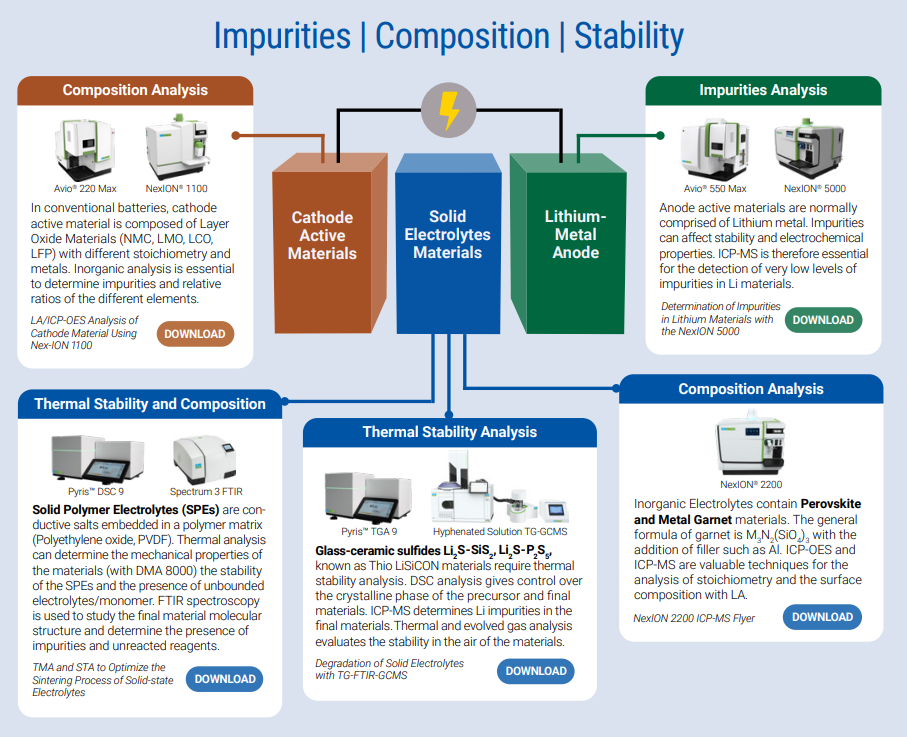
Solid State Batteries Material Analysis. Image Credit: PerkinElmer
Download Infographic
Thermal stability is crucial for battery safety and longevity. Could you explain how your differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) tools help manufacturers test and improve the thermal stability of their battery designs?
If we look at the process of putting batteries together, or even earlier, when building battery materials, it's important to understand how heat is dissipated as the battery is charged.
The DSC is used to analyze a material's thermal transitions, such as melting or glass transitions, while TGA, or thermogravimetric analysis, helps us understand the thermal degradation of battery materials. Over time, these materials tend to degrade, which is why batteries can only handle a certain number of charge and discharge cycles.
TGA helps us understand how materials break down and the rate at which this occurs with heat and temperature. It can also be coupled with infrared or gas chromatography to analyze the gases released. This is part of what we call evolved gas analysis, which occurs when the battery is heated.
By coupling TGA with IR, we can better understand these gases: Are they toxic or non-toxic? Since the electrolyte in the battery is often fluoride-based, heating can release highly toxic fluorinated gases. Understanding the chemical structure and concentrations of these gases is key to assessing the thermal stability of the battery and how it degrades during the charging cycle.
With increasing demand for sustainable and safer battery technologies, how does PerkinElmer support manufacturers in assessing environmental impact and ensuring regulatory compliance? Additionally, could you elaborate on how your analytical solutions aid in the recycling of battery materials, helping to close the loop in the battery lifecycle?
Yes, this is big. The EV market, similar to others, is migrating toward becoming more sustainable, aiming for net-zero emissions. PerkinElmer’s portfolio of analytical instrumentation helps just with that.
As the industry moves forward with different chemistries, it wants to ensure that the output of the battery improves—can it hold a bigger charge, can it charge faster, and so on? Our instruments play a key role in that process.
Now, as we move to battery recycling, it is an emerging market. You hear terms like 'black mass' or 'battery passports,' referring to different aspects of battery recycling.
When batteries reach the end of their life, larger components are first removed, and then the core is shredded and undergoes one of two processes: hydrometallurgy or pyrometallurgy. A key aspect is processing what is referred to as 'black mass.' At this stage, it's crucial to understand its composition. Does it only contain major elements like nickel, manganese, and cobalt, or are there impurities we need to identify before reintroducing the black mass into the recycling process?
Our goal is to close the loop by providing recyclers with analytical solutions to better understand the output from recycling so reclaimed metals can be reintroduced into the battery supply chain as efficiently as possible.
Battery testing often involves large-scale testing of multiple samples. How does PerkinElmer’s laboratory automation technology streamline the testing process in battery R&D, and what advantages does this bring to manufacturers?
Most of our instruments, whether in atomic spectroscopy, thermal analysis, or gas chromatography, are equipped with auto-samplers that allow the lab or chemists to load the samples and move on to other tasks. This really frees up a lot of their time to assume other day-to-day tasks.
Adding on to this, we have been advancing the industry by evolving sample preparation methods. Currently, the most common sample preparation techniques for batteries involve dissolving the samples using acids.
Over the past two years, we've introduced, through partnerships with a few companies, laser ablation technology coupled to ICP-OES or ICP-MS for battery materials testing. This allows the industry to work with solid samples directly, eliminating the need for chemical processes involving acids. This not only speeds up laboratory processes but also minimizes its environmental impact.
Analytical chemistry is critical in understanding the composition and performance of batteries. Could you elaborate on some recent advancements in PerkinElmer’s analytical chemistry tools and how they are helping researchers explore new material properties or improve battery efficiency?
That's fundamentally what we do—advancing the chemistries in batteries and adjacent markets.
If we look at the evolution of cathode materials, we have NMC and LFP, and now we're seeing LMFP, NCA, and LMNO, to name a few upcoming chemistries. These changes aren't driven solely by chemistry itself but also by the availability of raw materials.
For example, with NMC, the demand for high-grade nickel, coupled with a lack of active nickel mines and supply chain issues caused by geopolitical events, drove the industry to adopt alternative cathode chemistries.
Our instrumentation helps scientists and researchers work with these evolving chemistries, supporting advancements in cathode material development and the broader field of battery science.
From your experience, what are some of the most common challenges your clients in the battery industry face, and how do PerkinElmer’s solutions address these issues effectively?
I would say one of the biggest challenges facing the battery industry is managing the intricate and complex supply chain. To do so, you need instrumentations that are designed and set up with ready methods and everything required to accelerate your decision. We recently introduced the Avio ICP-OES lithium E-method package, a library of ready methods that eliminate the need for method development when testing for battery materials. Get e-Methods Pack pricing.
Essentially, we're offering a set of electronic methods on our atomic spectroscopy instruments, allowing startup labs that want to begin testing cathode materials, anode materials, and electrolytes to have a step-by-step guide. The methods are preloaded into the instruments, and we provide a detailed guide to needed consumables and sample handling.
This setup allows new companies entering the battery manufacturing space to have all their tools pre-configured in the instrument, with support from our team of scientists. It enables these companies to focus on their core work—improving their science—without worrying about instrument configurations, as that part has already been taken care of.
With new trends rapidly emerging in the battery industry, how is PerkinElmer adapting its tools and technologies to meet these evolving needs? Are there any upcoming products or innovations you’re particularly excited about?
We're constantly evolving our products and expanding our library of methods. For example, in atomic spectroscopy, we talked about the E-methods. Each year, we refresh that content with new methods based on what we have learned and feedback from the industry, focusing on what’s needed in terms of testing capabilities and capacities.
On the other side, one of the leading technologies we've seen a lot of success with is hyphenated systems. This system combines three instruments—TGA, IR, and GC-MS, all interconnected. I like to think of it as multiple analyzers working together, enabling the customer to advance their research faster. For example, if you want to know the moisture content in your battery blend or cathode, TGA will give you that information. If you also want to see what gases are being evolved from that mixture, the IR will provide part of that data. For a more detailed analysis, a sub-aliquot of the sample can be sent to the GC-MS.
This is just one example of a hyphenated system. Another could involve atomic spectroscopy. If you want to know what type of lithium electrolyte or lithium molecule is forming, a liquid chromatography-based separation can feed into the ICP-OES or ICP-MS, advancing our understanding of the process.
To summarize, I believe the innovation lies in coupling multiple instruments together, giving our users the ability to advance their science at a faster pace.
About Chady Stephan
Chady Stephan holds a Ph.D. in Analytical Chemistry from Université de Montréal. He joined PerkinElmer as an atomic spectroscopy application scientist. Since then, he has held various roles inside the organization in product management and business development. He currently directs a team of market managers focusing on core capabilities within focused end markets. He is a thought leader in elemental analysis, with over 30 peer-reviewed papers and book chapters.
For More Information...
You can download PerkinElmer's Battery Analysis Guide.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.