The Zeta Potential of a colloidal dispersion is a measure of the electrostatic repulsion between particles and can be used as an indicator of dispersion stability.
If all the particles in suspension have a large negative or positive zeta potential then they will tend to repel each other and there is no tendency for the particles to come together. However, if the particles have low zeta potential values then there is no force to prevent the particles coming together and flocculating.
Zeta potential is influenced by pH, ionic strength (the concentration and type of ions present) and the concentration of any charged molecules in the dispersant. The effect of the pH, or ionic strength of the medium or the concentration of an additive on the zeta potential can give information in formulating the product to give maximum stability.
Further information on zeta potential can be found in the technical note “Zeta Potential: An Introduction in 30 Minutes” (KB000734) available on the Malvern Panalytical web site.
The measurement of zeta potential can be performed on a suitable Zetasizer Nano instrument using the technique of laser Doppler electrophoresis. Further information on this technique can be found in the technical note “Measuring Zeta Potential: Laser Doppler Electrophoresis” (KB000606) available on the Malvern Panalytical web site.
Introduction to Isoelectric Point
The isoelectric point (IEP) is defined as the point of zero zeta potential. For a sample which is electrostatically stabilized, the IEP is often the point of least stability due to the repulsive forces being weakest. This may be important when considering the shelf life of a product, as normally the sample needs to be away from the IEP.
Figure 1 shows a typical plot of the zeta potential of a sample measured as a function of pH. In this example, the isoelectric point of the sample is at approximately pH 5.5. In addition, the plot can be used to predict that the sample should be stable at the extremes of pH.
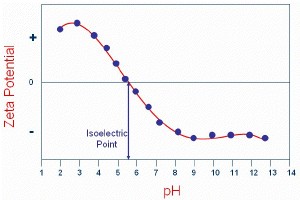
Figure 1. A plot of the zeta potential of a sample measured as a function of pH.
For example, at pH values less than 4, there is significant positive charge present. In addition, at pH values greater than 8, there is significant negative charge present. At these extremes of pH, the forces resulting from this electrostatic repulsion should be sufficient for the sample to resist flocculation.
In order to determine whether a sample has an IEP or not, the conditions of the sample need to be altered and the effect on the zeta potential monitored. This procedure can be carried out manually. However, automatic determination is less time consuming and more desirable and can be achieved by combining a Zetasizer Nano instrument with a Multi Purpose Titrator MPT2.
What is Multipurpose Titrator MPT2
The MPT-2 is an integrated system designed to automate changes in the composition of the sample and then transfer the sample to the optics unit for measurement of the size, zeta potential and intensity.
Figure 2 shows a typical titration schematic for the MPT2 linked to a Zetasizer Nano. The MPT2 has the ability for three titrants to be used to change the composition of the sample and can be programmed to perform the following titration types:
- pH
- Additive (linear)
- Additive (logarithmic)
- Dilution
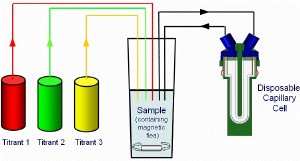
Figure 2. A typical titration schematic showing the ability to have three titrants for changing the composition of the sample and circulating it into the disposable capillary cell situated in the Zetasizer Nano.
The titrants used will depend upon the type of titration requested. For example, if the effect of changing the pH on the zeta potential of a sample wanted to be studied, the titrants would be acid and base. If the change in conductivity wanted to be studied, an appropriate salt would be used as a titrant in a logarithmic additive titration.
After the conditions have been changed, the sample is then circulated into the disposable capillary cell situated in the Zetasizer Nano for the measurements to be made. The measurement of particle size, zeta potential or the intensity of scattered light can be programmed depending upon which Zetasizer Nano instrument is used.
Isoelectric Point Examples
The following examples were all measured on a Zetasizer Nano ZS instrument in conjunction with an MPT2 at 20˚C. This temperature ensured that equilibration of sample viscosity was minimized as the bulk of the sample situated external to the Nano instrument was at a room ambient temperature of 20˚C.
Figure 3 shows the zeta potential measured as a function of pH for a titanium dioxide sample dispersed in deionised water. The titration conditions are defined in table 1. Two concentrations of HCl titrant were used to increase the accuracy of adjusting the pH around neutrality. The repeatability of the measurements at each pH increment was excellent and the sample had an isoelectric point at pH 3.05.
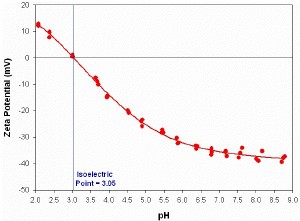
Figure 3. The zeta potential (in mV) versus pH titration for a titanium dioxide sample. Three repeat measurements were made at each pH point and the isoelectric point was determined to be at pH 3.05.
Table 1. Titration conditions for the zeta potential versus pH titration of titanium dioxide.
| Parameter |
Value |
| Titrant 1 |
0.25M HCI |
| Titrant 2 |
0.025M HCI |
| Titrant 3 |
0.25M NaOH |
| Start pH |
2 |
| End pH |
9 |
| pH Increment |
0.5 |
| Number of measurements at each titration point |
3 |
Table 2 summarizes the conditions used for a linear additive titration. A sample of magnesium hydroxide was titrated with FeCl3 to try and optimize dispersion conditions. A stock FeCl3 solution of 1000ppm was used as the titrant and 3 repeat measurements were performed at each titration point. The repeatability of the results was excellent and the magnesium hydroxide sample had an isoelectric point at 5.2ppm of FeCl3.
Table 2. Titration conditions for the zeta potential versus FeCl3 concentration for a magnesium hydroxide sample.
| Parameter |
Value |
| Titrant 1 |
1000ppm FeCl3 |
| Start additive concentration |
0ppm |
| End additive concentration |
20 |
| Additive concentration increment |
1 |
| Number of measurements at each point |
3 |

Figure 4. The zeta potential (in mV) versus FeCl3 concentration (in ppm) for a magnesium hydroxide sample obtained from a linear additive titration. Three repeat measurements were made at each FeCl3 concentration and the isoelectric point was determined to be at 5.2 FeCl3 ppm.
Conclusions
The determination of isoelectric points is important in understanding the stability of colloidal dispersions. The Multi Purpose Titrator MPT2 used in conjunction with a Zetasizer Nano instrument allows for the automatic determination of isoelectric points.
.png)
This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information on this source, please visit Malvern Panalytical.