A solute’s electrophoretic mobility is greatly affected by the solvent environment, especially parameters such as the solution pH, temperature, solute concentration, and ionic strength or formulation excipients.
Data implies that solute charge is predominant at low ionic strengths, causing repulsion through long range interactions. And at high ionic strengths, counter-ions increasingly shield these ionic interactions, typically causing a decrease in the repulsion of solute molecules.
This observation suggests that ion shielding supports short- and mid-range interactions, but decreases long-range charge-charge interactions. The increasing ionic strength of the solution raises the count of counter ions in the locality of the solute molecules, thereby causing decrease in mobility.
The objective of this analysis is to demonstrate the effect of decreasing mobility caused by increasing concentration of sodium chloride (NaCl) for the protein bovine serum albumin (BSA).
Mobility Measurements of High Salt Solutions
Historically, it is difficult or impossible to have mobility measurements for aqueous samples with conductivities near or above physiological saline conditions. If a solution conducts electricity, then applying the required electric for mobility measurements causes the current to traverse the solution.
This, in turn, triggers electrochemical redox reactions to cause electrolysis on the electrode surface, releasing gas bubbles which affect the driving electric field and sacrifice the light scattering signal utilized for mobility measurements.
The Möbiuζ (Mobius) flow cell is pressurized by Wyatt Technology's Atlas accessory for mobility measurements at high conductivities in order to suppress and decrease the overall volume of the existing gas bubbles.
The Möbiuζ cell, which is a closed-system, facilitates automation and high-throughput parallel measurement of hydrodynamics radius and electrophoretic mobility. A pre-programmed HPLC sequence provides 500 pL injections of the sample, thus enabling automated measurements of multiple samples conveniently.
Analysis and Results
The samples were prepared at 2 mg/mL into 50 mM phosphate buffered saline, in which the total concentration of NaCl varied between 50 and 2000 mM. This, in turn, covers a conductivity range between 5.6 mS/cm for the 50 mM Phosphate; 50 mM NaCl and 86.2 mS/cm for the 50 mM phosphate; and 2000 mM NaCl.
The total time taken for six measurements of each sample was 3 minutes in addition to 5-12 minutes taken to deliver the sample, halt flow, equilibrate, and then wash out the spent sample. Each sample was injected three times for a total of 18 measurements. The combination of low sample temperature (4 °C), low applied voltage (3.5 V) and short measurement times reduced electrolysis and sample degradation.
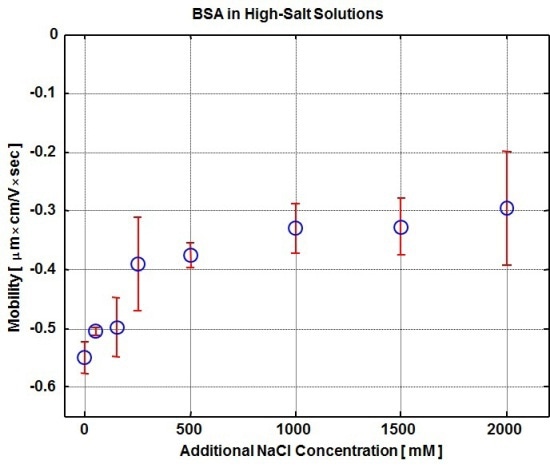
Figure 1. Plot of electrophoretic mobility vs. increasing salt concentration for 2mg/mL BSA injections measured at 4°C.
Plot of electrophoretic mobility versus increasing salt concentration for 2mg/mL BSA injections measured at 4°C is shown in Figure 1. At the lowest concentration of NaCl, i.e., at 50 mM, the magnitude of the measured mobility is very high with a value of -0.51 mm cm/Vsec. The mobility magnitude of BSA declines with increasing NaCl concentration and finally plateaus at a value of roughly -0.33 mm cm/Vsec.
The mobility remains close to this value for up to 2000 mM NaCl, where the corresponding conductivity is 86 mS/cm. The increasing ionic strength results in more counter ions associated with the solute molecule, causing screening of the molecular charge, which, in turn, reduces the electrophoretic mobility.
Conclusion
Automated analyses of high salinity samples are made possible, probably for the first time, through the use of the Möbiuζ flow cell coupled with the Atlas and auto sampler.
The versatility of the Möbiuζ hardware makes it a highly handy tool to measure the hydrodynamic radius and electrophoretic mobility for formulations and solutions at high ionicities. This, in turn, enables charge characterization of samples prepared in high-salt buffers.

This information has been sourced, reviewed and adapted from materials provided by Wyatt Technology.
For more information on this source, please visit Wyatt Technology.