Hemoglobin is the key protein in the red blood cells of mammals, playing the essential role in transporting oxygen from the lungs to respiring tissue in the body. Subsequent to the release of oxygen, carbon dioxide binds to hemoglobin to be released out of the body.
Instrumentation
Human hemoglobin is composed of four polypeptides, wherein the tetramer equilibrates with a dimer form based on the conditions. A combination of size-exclusion chromatography (SEC), multi-angle light scattering (MALS), Optilab DSP interferometric refractometer, and a miniDAWN detector is used for hemoglobin characterization, enabling to readily acquire data on absolute molar masses and molar mass distributions.
Analysis and Results
The hemoglobin molar mass is plotted against the elution time as shown in Figure 1. The molar mass across the peak is not consistent, which is probably owing to an equilibrium shift from a tetramer to a dimer that occurred during the chromatography run.
It may be difficult to differentiate regular peak extending from the small polydispersity of the sample by means of conventional column calibration. However, it is easier with the SEC-MALS measurement.
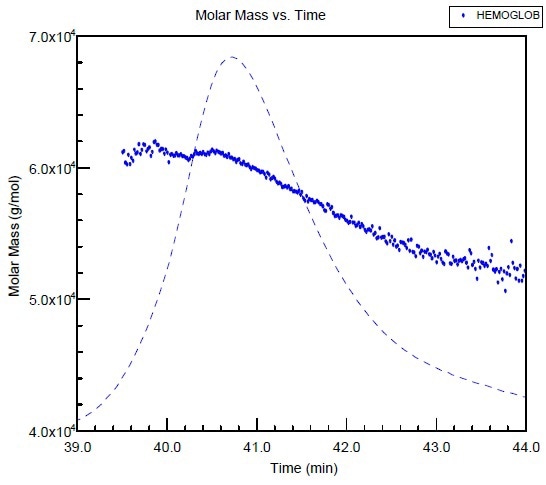
Figure 1. Molar mass versus elution time of Hemoglobin obtained from SEC using two columns and combined with MALS detection.
Bovine serum albumin (BSA), a standard protein, is typically utilized for column calibration. Figure 2 shows the overlay of the molar mass versus elution time plot of hemoglobin with that of BSA. Although the molar masses of the two proteins are almost in the same range, their shapes and corresponding elution times are completely different. However, BSA elutes well before hemoglobin as it has less compact structure when compared to hemoglobin.
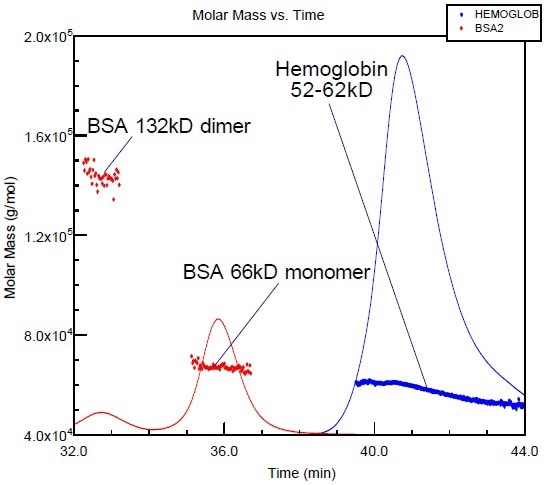
Figure 2. BSA and hemoglobin (proteins with similar molar masses) elute at different volumes, due to the more compact structure of hemoglobin.
Conclusion
If BSA is used as a calibration standard as in many laboratories, there is a possibility for the hemoglobin molar mass to be significantly underestimated. However, it is possible to avoid all of the assumptions and erroneous conclusions by coupling the chromatography with a miniDAWN or DAWN.

This information has been sourced, reviewed and adapted from materials provided by Wyatt Technology.
For more information on this source, please visit Wyatt Technology.