Feb 21 2001

Image Credit: Yothin Tenpet/Shutterstock.com
Article updated on 12/02/2020 by Gaea Miranda
Due to the electrical potential difference that develops when two dissimilar metals or alloys are connected together in an aqueous solution, the base metal will become anodic and the more noble metal will act as a cathode. When the noble metal is in effect, it is cathodically protected by the more reactive corroded metal.
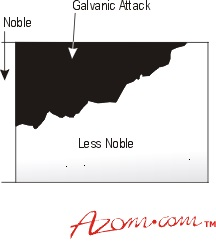
Figure 1. Galvanic attack
Galvanic Series
A galvanic series of metals and alloys could be organized through listing the noble and base materials in a specific corrosive environment. For example, the below table shows materials in seawater that are potentially corrosive in a galvanic couple.
Table 1. Simplified galvanic series for metals and alloys. The relative position in the series will depend on the corrosive environment and on the passivity of the surface of the metal or alloy.
| Noble (Cathodic) |
Base (Anodic) |
| Platinum |
Magnesium |
| Gold |
|
| Graphite |
|
| Titanium |
|
| Silver |
|
| Stainless steels |
|
| Nickel |
|
| Monel |
|
| Cupronickel |
|
| Tin bronze |
|
| Copper |
|
| Cast iron |
|
| Steel |
|
| Aluminum |
|
| Zinc |
|
An attack on the base metal will usually be more severe at the junction with the noble metal, but the extent of the damage will instead depend on the electrochemical differences between them. For example, the wider the separation in the galvanic series, the greater the attack on the base partner. The relative surface areas of the two metals exposed to the corrosive media and the nature of that media will also have an effect. When small surface areas of base metal are connected to much larger areas of noble materials, the attack on the base metal will subsequently be rapid.
This phenomenon was originally illustrated in 1761 through the first recorded example of the galvanic effect with the detachment of copper sheets from the hull of HMS Alarm. This was as a result of the attack on the iron nails, which had been used to attach copper to the timbers of the material.
Minimizing the Effects of Galvanic Attacks
Similar to other forms of corrosion, galvanic attacks can also be minimized through correct design. The use of galvanically compatible materials and electrical insulation between dissimilar materials can help ease problems surrounding galvanic attacks. Not coating the anodic surface to protect from pinhole damage is also useful, as this could give rapid local attack.
The galvanic effect is the reason why different phases and segregated regions in alloy microstructures will have varying resistance to corrosion. This effect is used efficiently when polished specimens are selectively attacked by etching in order to reveal and study microstructural features under the microscope. In stainless steels, Chromium (Cr)-depleted zones around Cr-rich second phases will be less noble. As such, they will be subject to highly localized attacks that can ultimately lead to interdendritic and/or intergranular forms of corrosion.