Delivering dry powders to the lungs and to the nasal cavity is gaining popularity in the pharmaceutical industry as both targets provide a large surface area for absorption, deliver drugs without affecting the gastrointestinal route, and avoid the patient acceptability problems related to intravenous administration.
Dry-powder inhalers (DPIs) provide an environmentally friendly option to metered-dose inhalers and need little or no patient cooperation because delivery is induced by the patient drawing breath. Stability is also a key benefit of dry formulations in both nasal and pulmonary delivery.
Nevertheless, the development of successful DPIs depend on engineering a powder blend capable of dispersing to a respirable size, typically below 5 µm, which facilitates the drug delivery solely by the inhalation effort of the patient.
One solution involves the use of ’carrier’ particles to optimize flow and dispersion behavior. However, the alternative ‘carrier-free’ solution reduces the amount of material deposited in the mouth and throat by means of sedimentation and impaction.
Developing Carrier-Free DPI Formulations
Active pharmaceutical ingredient (API) particles must have a size of around 5 µm or less for targeting the lung. At sizes below 10 µm, the tendency for particles to agglomerate is dramatically increased because of the exponential increase in the strength of inter-particle forces of attraction.
Therefore, it is necessary to modify the properties of fine API particles in order to minimize the cohesive forces between them. Reducing the contact area between particle surfaces, for instance by means of increased surface roughness, is one method. Minimizing particle surface energy is another approach.
Imaging Analyzes and Results
This article covers the analysis of three samples of salbutamol sulphate coated with different proportions of nano-sized L-leucine crystals using a PVD process in an aerosol flow reactor to explore the effect of varied proportions of L-leucine on the surface topology, particle shape and dispersion behavior of a DPI. The analysis also involved the characterization of a micronised salbutamol sulphate (MSS) sample for comparison.
Morphologi G3, an automated image analysis-based particle characterization system from Malvern Panalytical, was used to assess the particle morphology of each of the samples. Circularity data for the four samples is shown in Figure 1. Particles with circularity close to zero represent a more irregular shape, while circularities closer to one represent a 2D projection with an almost perfect circle.
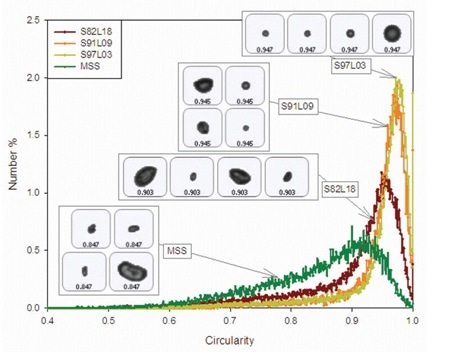
Figure 1. Circularity distributions for coated and uncoated samples of salbutamol, including example images of particles representing the mean circularity values.
The results demonstrate that circularity reduces with increasing L-leucine proportion for the coated samples. Conversely, the MSS sample shows the lowest overall circularity value and indicates the presence of considerable amount of elongated particles, as shown in Figure 1. SEM results concur that the surface topography and particle shape for the four samples are different.
Analysis of Drug Delivery Characteristics Using Laser Diffraction
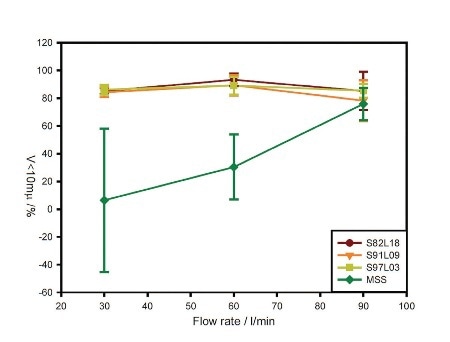
Figure 2. Flow-rate dependence of the volume of material below 10 µm in size for each formulation.
Spraytec, a laser diffraction system from Malvern Panalytical, was used to assess the aerosolization properties of both the coated and uncoated samples. A passive inhaler device was used to perform the tests for each formulation at 30, 60 and 90 L/min.
Both dispersed particle size and entrainment rate were analyzed as a function of flow rate. Gravimetric measurement of total emitted mass was performed by weighing the inhaler and capsule prior to and after each actuation.
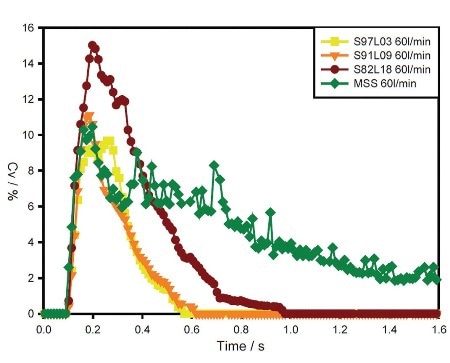
Figure 3. Concentration (Cv) profiles for S97L03, S91L09, S82L18 and MSS at the flow rate of 60 L/min.
For all three coated samples, the recorded data demonstrates good, reproducible dispersion to a respirable size at each flow rate, as illustrated in Figure 2. This observation is characteristic of device overload due to rapid powder entrainment.
Contrary to the coated samples, the MSS sample shows gradually increasing performance with increasing flow rate. Concentration profiles for the samples observed at 60 L/min flow rate emphasize differences in entrainment behavior, as depicted in Figure 3.
Table 1. Table showing average emitted mass for each formulation at 30, 60 and 90 L/min, as calculated over three device actuations.
| Flow rate (L/min) |
Emitted mass (mg) |
| S97L03 |
S91L09 |
S91L09 |
MSS |
| Average |
%RSD |
Average |
%RSD |
Average |
%RSD |
Average |
%RSD |
| 30 |
4.43 |
14.6 |
5.03 |
8.46 |
6.99 |
7.39 |
4.72 |
32.21 |
| 60 |
6.54 |
17.48 |
6.28 |
5.15 |
9.00 |
10.38 |
6.67 |
12.59 |
| 90 |
7.39 |
7.65 |
8.45 |
3.35 |
9.96 |
6.81 |
9.06 |
24.88 |
Entrainment is rapid for all three of the coated materials, providing a high initial particle concentration potentially favoring API delivery to the lungs. Emitted mass improves with flow rate for all samples, with the highest emitted mass recorded for the S82L18 in each case, as shown in Table 1.
Differences in emitted mass are likely to be higher at low flow rates, which deliver a comparatively small amount of energy for entrainment and dispersion of the dose. For the MSS sample, differences in emitted mass are higher at all flow rates, suggesting that break-up and dispersion of the MSS powder bed is comparatively slow and inefficient.
Conclusion
From the results, it is evident that the complementary techniques of image analysis and laser diffraction provide key insights to better understand the performance of the coated formulations for manipulating the variables that control drug delivery.
The results also demonstrate that coating the API with L-leucine nanocrystals is a workable method for altering the properties of the particles in order to optimize the dispersion of carrier-free formulations from passive DPIs.
The coating process minimizes the energy needed to disperse the material, thereby raising the level of fine particles and improving dose-to-dose reproducibility. Although all coated formulations demonstrated a high level of agglomerate dispersion, the characteristics of high L-leucine samples seem to be more favorable for improving delivery.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information on this source, please visit Malvern Panalytical.