Ongoing efforts to develop and implement affordable, easy-to-distribute vaccines have led to the development of ultra-low-cost electroporator with microneedle electrodes (ePatch) for SARS-CoV-2 vaccination.
Study: An ultra-low-cost electroporator with microneedle electrodes (ePatch) for SARS-CoV-2 vaccination. Image Credit: CKA/Shutterstock.com
While undoubtedly a further, robust tool in the global fight against COVID-19 and in preparing for future pandemics, this novel technology also offers specific benefits for developing countries or low-resource settings with limited access to trained personnel, due to its low cost and straightforward application mechanism.
DNA vaccines offer an array of benefits - they can be developed rapidly, are safe and cost-effective. Their key disadvantage lies in the need to employ costly, large and often unwieldy electroporation devices to ensure effective vaccination, presenting notable challenges when looking to implement effective mass vaccination regimes; particularly when working with hard-to-reach populations.
These limitations not only adversely affect patient and clinic access in low-resource settings, but these limitations are especially prevalent when dealing with pandemics like COVID-19, where the sheer number of electroporators required make these extremely challenging to effectively mass-produce and distribute at sufficient speed.
The COVID-19 pandemic has highlighted the vital need for a cost-effective, safe, efficient, and accessible electroporation platform able to administer DNA vaccines while being rapidly scalable enough to respond to outbreaks.
The new ePatch for SARS-CoV-2 vaccination is anticipated to cost less than $1 USD per unit to produce. It is provided in a handheld housing weighing less than 50 grams and under 20 cm3 in size; making it far smaller, lighter, and more cost-effective than current electroporators.
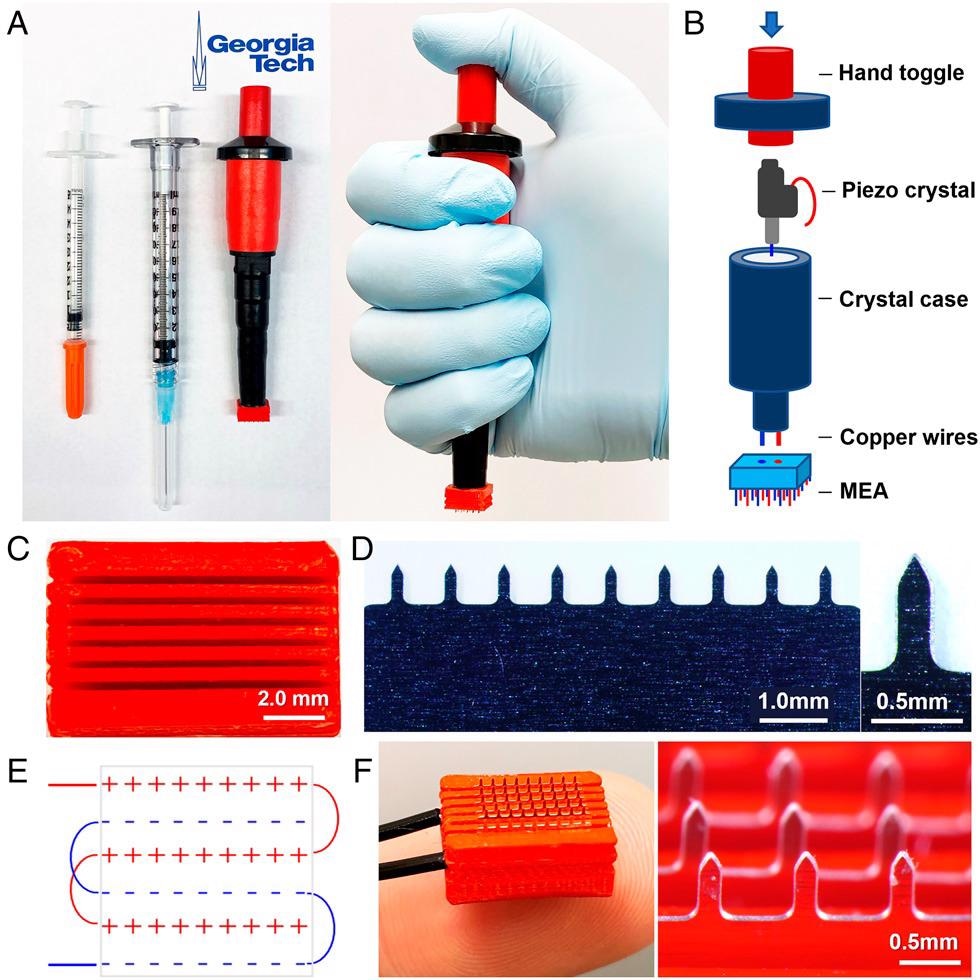
Design of electroporator with piezoelectric pulse generator and MEA. (A) The ePatch is shown with 0.5- and 1-mL syringes for comparison (Left) and shown being held in position before activation (Right). (B) Components of the ePatch. (C) The 3D printed insulating holder of microneedle electrodes in MEA to accurately position and electrically isolate microneedle electrodes of opposite polarity. (D) A row of stainless steel microneedle electrodes (Left) and a single microneedle (Right). (E) Diagram showing the configuration of the electrodes in an MEA. (F) Assembled MEA (Left) and magnified view of a section of the MEA in an assembled ePatch (Right).
Its minimal cost and compact size are ensured through the use of a thumb-operated piezoelectric pulse generator based on the design of a common household gas lighter, currently mass-produced in their millions.
The microneedle electrode array (MEA) used in the ePatch has been produced via lithographic etching technology and 3D printing – methods commonly used to manufacture components for a diverse array of disposable consumer products.
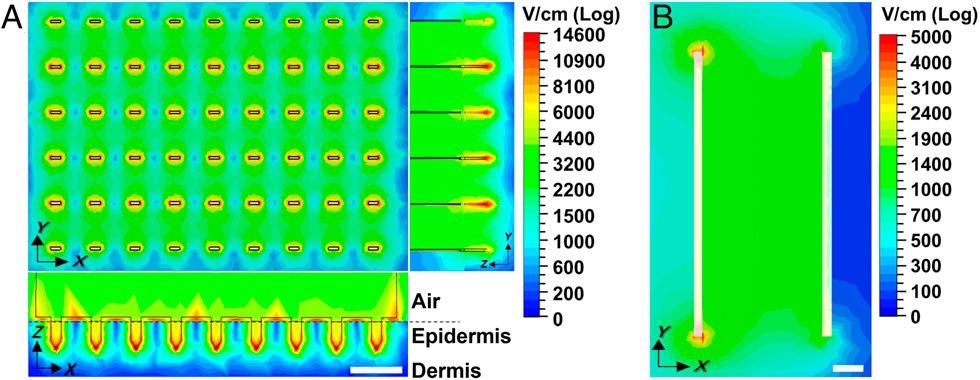
Electric field strength distribution in the skin is determined by computer simulation. Peak electric field strength is shown when applying a 300-V pulse-like those from the ePatch using an (A) MEA or (B) clamp electrode. Field strength distribution is shown in A from above the MEA (Upper Left) and as side views (Bottom Left and Right), and in B from above the skin. Dermal�epidermal junction is indicated by the dashed line. (Scale bar: 1 mm.)
The ePatch design does not require any battery or other power supply, making it highly portable and applicable in a wide range of settings.
The design of the MEA is particularly impressive, comprised of six rows of stainless steel microneedles. These microneedles measure 650 μm in length, divided into 200 μm by 50 μm cross-sections and tapering to a sharp tip mounted in a 3D-printed, polylactic acid insulating holder.
Each row of electrodes in the MEA is required to exhibit the same electrical polarity. These electrodes are placed in extremely close proximity - nine microneedles each separated by 0.8 mm spacing within each row, with rows 0.9 mm apart. This close spacing is important in facilitating the electric field strength required to ensure electroporation via microsecond pulses.
Positive and negative terminals are connected via wire to both the piezoelectric pulse generator and the MEA.
When used to administer a vaccine, the pulser emits microsecond, bipolar, oscillatory electric pulses while the MEA targets delivery of high electric field strength pulses to the epidermis of the skin.
The inexpensive nature of the ePatch means that it could be entirely disposed of following a single use, but it is possible to further reduce costs and minimize waste by reusing the piezoelectric pulser and replacing the MEA after each use to ensure safety and improve hygiene.
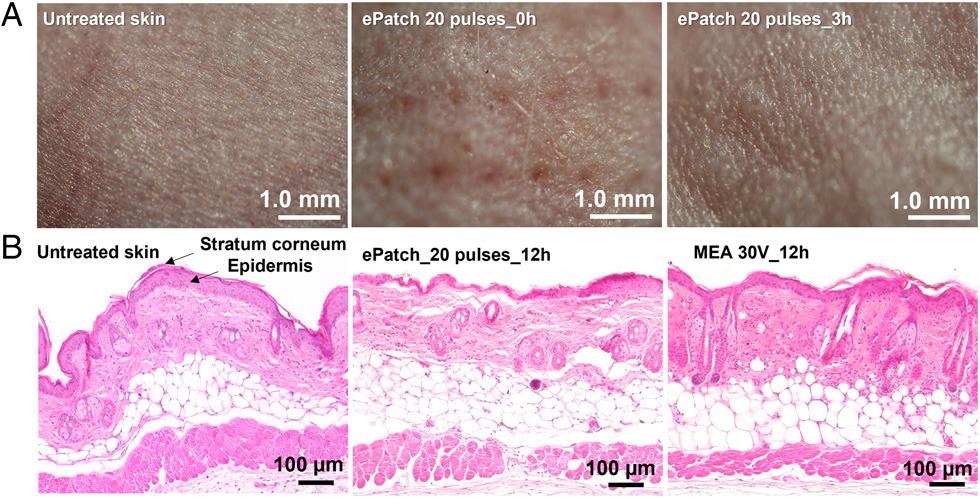
Histological examination of skin after electroporation in vivo. (A) Representative images obtained by stereo microscope are shown for untreated mouse skin and skin 0 h and 3 h after electroporation using 20 pulses by the ePatch in vivo. (B) Representative images obtained by brightfield microscopy of untreated mouse skin and skin electroporated with pulses by MEA combined with conventional millisecond electroporator (30 V, 55 ms) or ePatch with 20 pulses of microsecond duration. The skin was harvested 12 h after electroporation and H&E stained for examination.
Testing of the ePatch in rats and mice confirmed that it was able to selectively transfect cells in the epidermis via microsecond pulses, while demonstrating no evidence of lasting damage to skin.
The study was successful in highlighting the vast potential for microsecond, oscillating pulses delivered via an ultra-low-cost piezoelectric power source and an MEA with sub-millimeter microneedle electrodes for use in DNA vaccination against SARS-CoV-2.
While it is widely recognized that studies into DNA vaccination necessitate the use of a sophisticated and expensive powered electroporation device which uses large needle electrodes to penetrate skin or muscle; it is also widely reported that this complexity can severely limit rapid and widespread access to developed vaccines.
This novel ePatch addresses this barrier to the widespread application of DNA vaccination thanks to its inexpensive, straightforward, battery-free, and user-friendly delivery system comprised of components already manufactured at scale.
Initial studies have highlighted that this approach offers enhanced immune responses to SARS-CoV-2 DNA vaccination, exhibiting robust humoral immune responses and viral neutralization. The ePatch method also exhibited good tolerability with no apparent safety issues.
The ePatch also offers excellent potential for use with a range of other pathogens, potentially speeding up development, lowering costs, and improving worldwide access to life-saving vaccines.
Journal Reference:
Dengning Xia, Rui Jin, Gaurav Byagathvalli, Huan Yu, Ling Ye, Chao-Yi Lu, M. Saad Bhamla, Chinglai Yang, Mark R. Prausnitz. (2021). An ultra-low-cost electroporator with microneedle electrodes (ePatch) for SARS-CoV-2 vaccination. Proceedings of the National Academy of Sciences Nov 2021, 118 (45) e2110817118; DOI: 10.1073/pnas.2110817118. https://www.pnas.org/content/118/45/e2110817118.short
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.