Two new options for the Parsum IPP-70 in-line particle size measurement probe, supplied by Malvern Panalytical, are designed to simplify regulatory compliance for pharmaceutical applications. Full details will be available at the Malvern Panalytical booth (#1205) at this year’s Interphex event in Philadelphia, where the company will also present the Insitec Pharma Voyager system for mobile on-line particle size analysis, the industry leading Mastersizer 2000 and the SyNIRgi near infrared chemical imaging (NIR-CI) system.
The Parsum IPP-70 provides real-time particle size analysis, and is used in pharmaceutical manufacturing to monitor and control granulation, coating and spray drying processes. The first of the new Options allows for the provision of Installation/Operational Qualification (IQ/OQ) documentation together with a verification kit for checking measurement accuracy. In addition the second offers a unit with highly polished contact surfaces, and includes material traceability documentation.
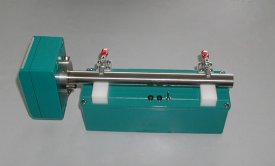
The Parsum IPP-70 delivers size and velocity measurement for particles, granules and pellets from 50 to 6000 microns in diameter. Light and compact, it can be used to monitor gravity fed, pneumatically conveyed and fluidized streams. It is inserted directly into a process line or unit, and is easily integrated with existing systems for automated control. An intrinsically safe version is also available (IPP-70-SE).
The verification kit supplied with Option 1, when used with new software (version 7.1), allows calibration of the whole measurement system – both probe and measurement PC. Calibration is achieved by measuring the diameter of three pins, of certified dimension, mounted on a rotating disc. Option 2 is particularly suitable for fouling duties and/or applications requiring high levels of hygiene.