May 30 2008
Researchers at the National Institute of Standards and Technology (NIST) have shown for the first time that a single layer of molecular “salve” can significantly soothe the stresses affecting clean metal surfaces. The discovery, revealed in a new paper, may help scientists to understand the factors that influence surface stress, which is important in a broad array of applications from chemical and biological sensors to semiconductor manufacturing and metal plating.
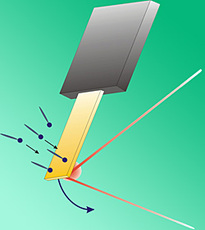 NIST researchers measured the surface stress of a monolayer film on gold by measuring the changing curvature of a gold-coated glass cantilever as molecules of mercaptobenzoic acid were deposited on the gold. The change in curvature was detected by the shifting reflection of a laser beam impinging onto the back side of the glass.
NIST researchers measured the surface stress of a monolayer film on gold by measuring the changing curvature of a gold-coated glass cantilever as molecules of mercaptobenzoic acid were deposited on the gold. The change in curvature was detected by the shifting reflection of a laser beam impinging onto the back side of the glass.
Because the atoms on a clean metal surface are not bound on all sides, they are much more strongly bonded to each other than to the atoms beneath them. Atoms in a block of metal are like a big family, relaxed when surrounded by kinfolk. But when the metal is cut, the atoms exposed at the surface cling tighter to the siblings at their sides and draw closer together. That creates surface stress and causes the edges to curl and pull in toward the center of the surface.
Materials scientists generally believed that a single layer of molecules coating the surface would reduce the stress, but no tests had ever been performed to determine whether or not that actually happens. NIST researchers devised an elegant, highly sensitive experiment to measure the phenomenon using a 6-cm long, 0.3-cm wide and approximately 100-micron-thick gold-coated glass cantilever and a “salve” of mercaptobenzoic acid, a carbon-based sulfur-containing compound used for manufacturing such products as pharmaceuticals and agricultural chemicals. The “salve” forms a well-organized single layer (monolayer) on gold, and it forms a model system for measuring variations in surface stress. The team repeatedly deposited and removed the monolayer and monitored the curvature of the glass with a laser as the stress increased and decreased. The technique enabled them to record forces of less than 50 micronewtons per meter.
According to Chris Zangmeister, an author of the study, in addition to confirming that the application of a monolayer did reduce surface stresses, the team also discovered that the longer the molecules were allowed to sit the more comfortable they became with their new surroundings. As the monolayer became more comfortable, it became more stable, and the atoms in the metal began to adopt the molecules into the family, which substantially reduced the surface stresses.
The findings provide a deeper understanding of the forces at work at the interface of molecules and surfaces. Most notably the discovery could be used to create a new generation of chemical and biological sensors. Zangmeister says that these sensors would use molecular monolayers deposited on metal surfaces that are manufactured to react in the presence of chemical or biological agents in the environment. The activation of the monolayer would provide a proportional response to the amount of the substance it was designed to detect, which would result in a quantifiable decrease in the tension of the cantilever.
The findings appeared online in Electrochimica Acta in December and will appear as an invited paper in a special print issue of that same publication.