Nov 11 2008
The breakthrough, reported in this week's edition of the journal Nature Materials, also came with a surprise. By devising a way to watch individual molecules react with a single nanoscale particle of gold in real time, researchers confirmed that some gold particles are better at increasing the rate of a chemical reaction than others, but they also found that a good catalyst sometimes spontaneously turns bad.
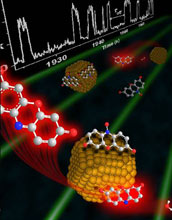 Nanoscale metal particles naturally have a wide variety of shapes and sizes and chemists long suspected that some particles work much better than others when it comes to catalyzing chemical processes. Researchers at Cornell University recently confirmed the hypothesis and discovered that some nanoparticles randomly change from good particles to bad particles.
Credit: Courtesy of Cornell University
Nanoscale metal particles naturally have a wide variety of shapes and sizes and chemists long suspected that some particles work much better than others when it comes to catalyzing chemical processes. Researchers at Cornell University recently confirmed the hypothesis and discovered that some nanoparticles randomly change from good particles to bad particles.
Credit: Courtesy of Cornell University
Understanding why these particles change and how to stabilize the "good" particles may lead to solutions for a wide range of problems such as the current global energy challenge.
Source: National Science Foundation