Nov 25 2008
You never know where basic research may lead. For decades materials scientists have been experimenting with a corkscrew-like polymer structure called a gyroid. Now an international team of researchers has shown that the gyroid structure can be used to "self-assemble" a low-cost photovoltaic cell.
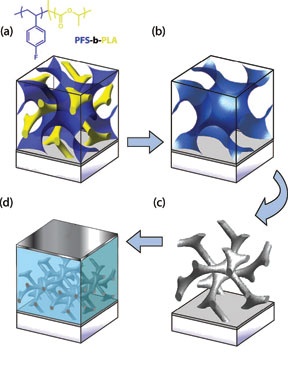 A self-assembled solar cell begins with one of two polymers forming a "gyroid" shape while the other fills in the space around it. The inner polymer is dissolved away to create a mold that is filled with a conductor of electrons. The outer polymer is then burned away, the conductor is coated with a photosensitive dye, and finally the surrounding space is filled with a conductor of positive "holes." A solar reaction takes place at all the interfaces throughout the material, and the interlocking gyroid structure efficiently carries away the current. Provided/Wiesner Lab
A self-assembled solar cell begins with one of two polymers forming a "gyroid" shape while the other fills in the space around it. The inner polymer is dissolved away to create a mold that is filled with a conductor of electrons. The outer polymer is then burned away, the conductor is coated with a photosensitive dye, and finally the surrounding space is filled with a conductor of positive "holes." A solar reaction takes place at all the interfaces throughout the material, and the interlocking gyroid structure efficiently carries away the current. Provided/Wiesner Lab
The idea could lead to more economical solar collectors and more efficient fuel cells.
The prototype is a variation on the Gräetzel solar cell, which uses an organic dye sandwiched between two conductors. Forming the conductors into an interlocking corkscrew allows current to be transported out efficiently. The team's first cell, made in a thin film 400 nanometers thick, has a conversion efficiency ranging from 0.7 to 1.7 percent -- low compared with silicon-based photocells, but "amazing" for such a thin film, said Ulrich Wiesner, the Spencer T. Olin Professor of Materials Science and Engineering at Cornell.
"The next step is to make it thicker" so more of the light falling on it can be captured, he said. "We hope that we will eventually get efficiencies that rival silicon-based devices." Currently available silicon-based solar cells convert about 15 percent of the energy of the light falling on them to electricity, although some new designs promise higher efficiencies.
The work by Wiesner and scientists at the Universities of Cambridge and Oxford in Britain, the Freiburg Institute for Advanced Studies in Germany, Institute Curie in France and the University of Minnesota, Minneapolis, is described in the online version of the journal Nano Letters and will appear in a forthcoming print issue.
The gyroid is one of four known structures that can be "self-assembled" at the nanoscale using block copolymers. A polymer is made up of organic molecules that chain together to form a solid or semisolid material. A block co-polymer is made by joining two molecules at the ends so that when they chain together, one forms a nanoscale pattern of repeating geometric shapes -- planes, spheres, cylinders or the twisted jungle-gym gyroid -- while the other fills the space in between.
Several years ago Wiesner's group showed that a block copolymer structure could be made in which just one of the polymers was a conductor of ions (charged atoms) and that the interconnected gyroid structure was the most efficient conductor.
To use this efficiency in a solar cell the researchers assembled a copolymer gyroid film, then dissolved away just the corkscrew part of the structure, leaving a corkscrew-shaped mold that they filled with titanium oxide. Heating then burned off the other polymer part and crystallized the titanium oxide into a solid structure that conducts electrons. This was coated with a light-sensitive dye, and finally the space around it was filled with a material that conducts "holes" (positive charges).
When light strikes the dye it knocks loose electrons, which flow into the titanium oxide framework, while the holes left behind flow into the other conductor. Electrodes above and below the film carry off the resulting current.
The secret of a solar cell, Wiesner explained, is that the electron-hole pairs must find the interface between the two conductors within 10 nanometers (about the width of 30 atoms) so they can separate and flow away, or they will recombine.
"This is why block copolymers are exciting," Wiesner said, "because that is the characteristic length scale of separation of the two blocks."
The experiments were done at Cambridge while Wiesner was on sabbatical there. Marleen Kamperman, a graduate student in Wiesner's group, used the facilities of the Cornell High-Energy Synchrotron Source (CHESS) for X-ray diffraction studies and interpreted transmission electron microscope images to show that the devices actually had the predicted gyroid structure.
The research was supported by the National Science Foundation, the Department of Energy, the Engineering and Physical Sciences Research Council of the United Kingdom, the Royal Society and the Leverhulme Trust.