May 6 2009
The cleanliness of high-performance safety-relevant components is an essential factor for reliability and a long lifetime. Leica Microsystems developed a microscope system for measuring impurities in cleaning liquids for micromechanic and motor components during production which has been used with great success for quantitative cleanliness analysis in the automobile and many other industries. New quality standards are putting increasing pressure on manufacturers to apply this technique. Leica Microsystems has now announced the launch of the successor product, Leica Cleanliness Expert, which offers further benefits for users.
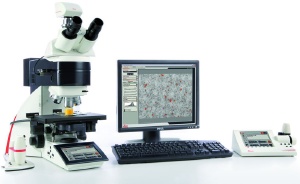
Leica Cleanliness Expert is an all-in-one system consisting of a fully automated incident light microscope, a digital camera and an image analysis system. Fibers and particles are detected in the live im-age and reflecting particles can be differentiated from non-reflecting ones. The system is able to fully reproduce any number of measurement parameters and microscope and camera settings, and all the changes are documented in the report. Also, a new scanning algorithm allows the user to detect any size of fibers and particles and reclassify them in the list of results. The convenient user interface in the familiar Leica Application Suite (LAS) design guides the user through all the main functions, making work quick and easy. Leica Cleanliness Expert offers ultra precise analysis leading to ultra reliable re-sults.
Leica Microsystems is a leading global designer and producer of innovative, high-tech, precision optical systems for the analysis of microstructures. It is one of the market leaders in each of its business areas: Microscopy, Confocal Laser Scanning Microscopy with corresponding Imaging Systems, Specimen Preparation, and Medical Equipment. The company manufactures a broad range of products for numerous applications requiring microscopic imaging, measurement, and analysis. It also offers system solutions for life science including biotechnology and medicine, research and development of raw materials, and industrial quality assurance. The company is represented in over 100 countries with 10 manufacturing facilities in 8 countries, sales and service organizations in 19 countries and an international net-work of dealers. The international management is headquartered in Wetzlar, Germany.