Jan 14 2010
The precious metal gold is the material of choice for many technical applications because it does not corrode - and because it also has interesting electrical, magnetic, and optical properties. Gold is thus one of the most important metals in the electronics industry, miniaturized optical components, and electrochemical processes.
 © Wiley-VCH
© Wiley-VCH
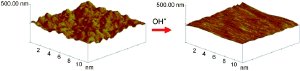
In these applications, it is extremely important that the surface of the gold be completely clean and smooth. However, conventional processes not only "polish" away the undesirable irregularities, but also attack the gold surface. Fritz Scholz and a team from the Universities of Greifswald (Germany) and Warsaw (Poland) have now discovered a technique that can differentiate between the two. As the scientists report in the journal Angewandte Chemie, hydroxyl radicals (OH radicals) rapidly remove all tiny protrusions on mechanically polished gold surfaces, leaving behind an extremely smooth surface.
The researchers treated gold surfaces with Fenton's reagent, which is a mixture of hydrogen peroxide and iron(II) salts that releases OH radicals. It is also used to degrade organic impurities in the purification of waste water. "Actually, it was not expected that the radicals would attack a polished pure gold surface," says Scholz, "because gold is notoriously difficult to oxidize." The experiments demonstrated that the hydroxyl radicals oxidize gold very well, though measurable dissolution continues only as long as there are still bumps on the gold surface. Though these results seem contradictory at first glance, the researchers explain that the reaction of the radicals with the highly ordered gold atoms of the completely smooth surface produces a stable layer of gold oxide, which can be reduced back to elemental gold without a significant loss of material. In the protrusions, however, the gold atoms are less ordered and very reactive. During the oxidation, they detach themselves from the atomic structure.
"Because the protrusions are selectively removed, our method is very interesting for polishing gold surfaces for industrial applications," says Scholz. The process may also find a use in medical technology: gold is used to replace teeth, in tissues for reconstructive surgery, and in electrode implants, such as those used for implanted hearing aids. These release tiny amounts of gold, which enters into the surrounding tissue. This apparently occurs because of an immune reaction that results in the formation of OH radicals or similar species. Pre-treatment of gold implants with Fenton's reagent could inhibit this release of gold into the body.