Apr 18 2010
Oxygen is the only elemental molecule which carries an electronic magnetic moment. As a consequence, the different solid phases encountered on cooling show various degrees of magnetic order, and similar behavior is expected under compression.
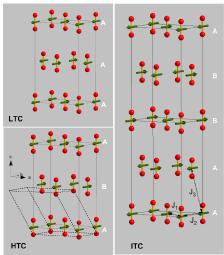 Magnetic structures in ä-O2. Upper Left: the low temperature commensurate (LTC) structure. Lower left: the high temperature commensurate (HTC) phase. Right: the intermediate commensurate (ITC) phase. Stability fields of the three phases are shown in Fig. 1. The unit cell of the low-pressure ä-phase (dashed) illustrates the close structural relationship between the two phases, having identical magnetic structures. ‘‘A’’ and ‘‘B’’ denote magnetic stacking sequence, J1, J2, and J3 the intra- and interplane exchange parameters.
Magnetic structures in ä-O2. Upper Left: the low temperature commensurate (LTC) structure. Lower left: the high temperature commensurate (HTC) phase. Right: the intermediate commensurate (ITC) phase. Stability fields of the three phases are shown in Fig. 1. The unit cell of the low-pressure ä-phase (dashed) illustrates the close structural relationship between the two phases, having identical magnetic structures. ‘‘A’’ and ‘‘B’’ denote magnetic stacking sequence, J1, J2, and J3 the intra- and interplane exchange parameters.
In an experiment lead at the ILL, at the powder diffractometer D20, using ILL's "Paris-Edinburgh" high pressure cell together with its cryogenic equipment, scientists Stefan Klotz and co-workers gathered neutron diffraction data which reveal the magnetic ordering under high pressure in the ä (so-called ''orange'') phase, i.e., in the range 6-8 GPa and 20-240 K. They showed that ä-O2 contains orders in total three different magnetic structures, all of them being anti-ferromagnetic and differing in the stacking sequence of magnetically ordered O2 sheets along the c axis. This structural diversity can be explained by the quasi-two-dimensional nature of ä-O2 and the strong orientation dependence of the magnetic exchange interaction between O2 molecules. The results show that ä-O2 is a room temperature antiferromagnet.
'The ILL is the only place in the world where this work was possible', says Dr Klotz.