We use a switch to turn lights off and on; however, light can also act as a switch itself, for example when molecules change their structure upon irradiation. Photoswitchable molecules are potentially interesting for use in holographic data storage, as molecular switches for nanomachines, or for switching biological functions in the biosciences.
In order to tailor these molecules for different applications, it is necessary to have a comprehensive understanding of the underlying reaction mechanisms. A team led by Nobel Laureate Ahmed Zewail and members of his group at Caltech in Pasadena (California, USA) now reports in the journal Angewandte Chemie about their use of electron diffraction studies to observe a photoswitchable molecule in the process of “switching”.
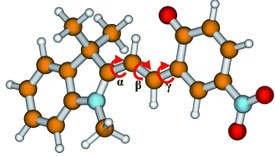
The molecule under examination was a complex ring system that switches between a closed form and an open form upon irradiation with UV light. In the closed spiropyran form it consists of two planar fused ring systems that form two orthogonal planes. When irradiated, a bond is broken to open a single ring. In this open merocyanin form, both units of the molecule are only connected through a bridge made by three bonds. Each of these bonds can theoretically have one of two spatial arrangements, which are designated as cis and trans. Furthermore, this molecule contains a nitro group (-NO2), which allows it to enter into two different electronic states—singlet or triplet—when excited by light.
Which form does it choose? This is what the researches wished to determine in order to study the reaction mechanism. To do this, they used a method known as laser-desorption electron diffraction. In this technique, a sample is heated and vaporized by laser so rapidly that the sample molecules do not have time to decompose. The isolated molecules are then bombarded with electrons. The electrons are diffracted by the atomic nuclei of the molecule, which results in a characteristic diffraction pattern. The scientists recorded diffraction patterns 100 nanoseconds before and after excitation with UV.
By using theoretical model calculations, the researchers were able to interpret these diffraction patterns. The result: “Ring opening leads primarily to the cis-trans-cis structure,” according to Zewail, “while competing, non-irradiative paths lead to other structures, such as the closed forms in their triplet and singlet ground states.”
“Our results demonstrate the enormous capability of the electron diffraction technique to solve such complex, nanometer-scale structures with minimal symmetry,” says Zewail.