Scientists at the Lawrence Berkeley National Laboratory (Berkeley Lab) of the U.S. Department of Energy (DOE) were the first to observe structural transitions inside a single copper sulfide nanocrytal, a semiconductor material.
The research team studied structural variations in a copper sulfide nanocrystal during its transition from low to high-chalcocite solid-state phases by utilizing transmission electron aberration-corrected microscope, TEAM 0.5.
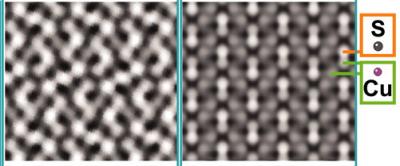 Fast Fourier transform pattern and high resolution TEM images of the low-chalcocite (green) and high-chalcocite (red) domains in a copper sulfide nanorod.
Fast Fourier transform pattern and high resolution TEM images of the low-chalcocite (green) and high-chalcocite (red) domains in a copper sulfide nanorod.
The study helped in understanding the structural changes in solid materials at the interface between an electrolyte and an electrode, or the ion transportation that occurs inside the electrodes during the discharging or charging process of batteries. The results of the study will help in developing next-generation energy technologies.
Paul Alivisatos, who serves as director at Berkeley Lab and leads the research team, stated that the defects found in the nanorod crystal are the influential factors behind the structural transformation dynamics. The study recommends methods for helping or repressing these structural transformations in order to develop materials with unique and controlled phases, he added.
During temperature changes, nanoscale solid material can switch between two more stages in its crystal structure. For instance, copper sulfide nanocrystal can be transitioned from an intricate hexagonal pattern called the low-chalcocite phase to a relatively simple hexagonal pattern called the high-chalcocite phase.
Alivisatos stated that the energetic barrier of nanoscale systems to their structural transition expands with their size. Phase transition theory explains that a solid crystal will vary between two equilibrium patterns close to the phase transformation point prior to attaining a stable structure. This transformation region widens in nanocrystals. To study this theory, the research team observed the expected fluctuations of copper sulfide nanorods under the TEAM 0.5 microscope’s electron beam.
According to Haimei Zheng, a member of the research team and a Berkeley Lab chemist, the research team will study structural modifications of nanoparticle catalysts and ion transportation within the battery materials that vary at the interface of the electrolyte and electrode.