New York University Researchers have created new materials with empty space on the inside; in effect they have developed molecular flasks, which could control unwanted and desired chemical reactions.
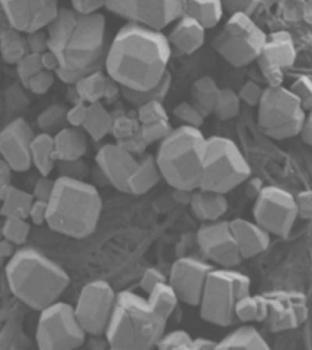 This is a scanning electron microscope image of a new material that self-assembles into a polyhedron using the attractive interactions associated with hydrogen bonds. The shapes then further organize into a crystal lattice that resembles a porous structure called zeolite, an absorbent material with many industrial uses. Credit - Michael D. Ward, New York University
This is a scanning electron microscope image of a new material that self-assembles into a polyhedron using the attractive interactions associated with hydrogen bonds. The shapes then further organize into a crystal lattice that resembles a porous structure called zeolite, an absorbent material with many industrial uses. Credit - Michael D. Ward, New York University
Chemical reactions happen everywhere in our daily life such as reactions that include rusting, burning or even the batteries that make use of chemical reaction for supplying electricity. Controlling these chemical reactions or even promoting the desirable and limiting the unwanted reactions is also very challenging for the researchers.
According to Mike Ward, who is from the Department of Chemistry in the NYU, his team had created self-assembling cages, which could house other compounds inside them and ultimately facilitate the isolation of certain chemical reactions both inside and outside the cage. This research was reported in the Science Journal dated 22nd July. Ward further mentioned that they had created these cages for separating chemicals on the basis of size and even carrying out reactions within well defined cages for easier control over the reaction products and the chemical reactivity.
These frameworks would also be perfect for housing a series of guest molecules, which would produce materials with new magnetic or optical properties. These molecular hotels take the shape of a truncated octahedron, which is one of the 13 Archimedean solids and future research projects would also try to develop other types of Archimedean solids and also utilize the truncated octahedron for housing a variety of functional molecules. Michael Scott, who is the Program Director at NSF’s Division of Materials Research, has stated that the Ward group had made use of some very simple chemical precursors and geometric design principles for the construction of reasonably sturdy materials, which include similar sized and shaped cavities. Chemists would get several opportunities for isolating unstable molecules, separating important chemical compounds and catalyzing unknown reactions.