Magnetic semiconductor research has shown the unique behavior of dilute magnetic semiconductors (DMS). The University of Science and Technology of China laboratory discovered the possibility of doping manganese (Mn) into zinc sulfide (ZnS) in order to obtain magnetic semiconductors.
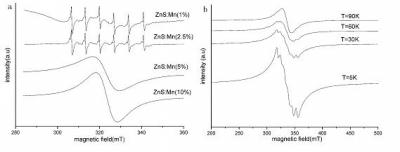 This shows (a) Room temperature ESR spectra of ZnS:Mn; and (b) Low temperature ESR spectra of ZnS:Mn (20%). Credit: ©Science China Press
This shows (a) Room temperature ESR spectra of ZnS:Mn; and (b) Low temperature ESR spectra of ZnS:Mn (20%). Credit: ©Science China Press
The evaluation of ferromagnetism in transition metal-doped semiconductors such as indium arsenide and gallium arsenide doped with Mn was first performed by Hideo Ohno and his team at the Tohoku University, Japan. Following this hypothesis, the researchers made efforts to obtain semiconductor materials doped with various transition metals showing ferromagnetic properties.
Determination on metal-doped semiconductors was conducted by a group of researchers from Hefei National Laboratory for Physical Sciences at the Microscale at the University of Science and Technology of China. Their work entitled ‘Structure Characterization, Magnetic and Photoluminescence Properties of Mn-Doped ZnS Nanocrystalline’ was published in SCIENCE CHINA Physics, Mechanics & Astronomy, 2012. The hypothesis shows that Mn-doped ZnS (ZnS:Mn) exhibits paramagnetic behavior and therefore cannot function as a DMS.
The electron spin resonance (ESR) spectra of nanocrystalline ZnS:Mn demonstrates that a typical sextet centered at a g-value of 2 integrates with the allowed (Äms=±1, ÄmI=0) magnetic dipole transitions that exists in between the hyperfine-split Zeeman levels of the 6S5/2’s Mn2+ 3d ground state electrons, at low concentrations of Mn. Following the interaction between the S=5/2 spin of the unpaired 3d electrons along with I= 5/2 spin of the 55Mn nucleus, a hyperfine structure results showing that Mn ions are distributed in the ZnS nanocrystalline lattice. At increased concentrations of Mn, the ions attain the ZnS crystal lattice, upon integration, increasing the dipole-dipole interaction and reducing the Mn-Mn atomic distance. The hyperfine structure therefore integrates into a single broad resonance. Even at low temperature, the ESR experiments show that ZnS:Mn is paramagnetic and therefore not ideal for DMS.