As more industries begin to comply with guidelines and regulations set forth by governing bodies like the U.S. Food and Drug Administration, the efficient management of liquid and gas chromatography (LC/GC) instruments and analytical data is essential. LabSolutions DB Software from Shimadzu Scientific Instruments offers LC/GC laboratories fast and secure management for their LC/GC data.
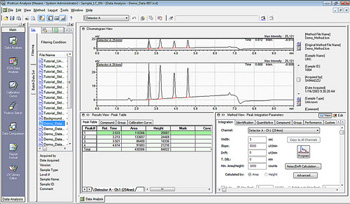
LabSolutions DB integrates a data management function with LabSolutions LC/GC control and analysis software. Optimally configured for PC-based laboratories, LabSolutions DB can be connected to up to four LC/GC instruments for simultaneous use. The integrated database is compliant with electronic records and electronic signature (ER/ES) regulations.
LabSolutions DB's project management function enables management suited to tasks and system operations. It improves the efficiency of data searches and management tasks by enabling equipment and user management, security policy and data processing to be set on a project-by-project basis.
The database allows multi-data report creation using Microsoft Excel. This enables users to coordinate report creation with scheduled analyses, making it possible to quickly create reports when testing ends.
Shimadzu offers LabSolutions DB as part of a scalable solution for laboratories that need ER/ES compliance but lack the IT staff required for full client/server installations.