Light absorbing materials demonstrate poor durability as they frequently degrade or overheat over time. This is one of the reasons that affect the widespread adoption of solar energy. To address these challenges, a research team comprising Dr. Mikhail Zamkov of Bowling Green State University and colleagues has developed a technique to produce two inorganic nanocrystals that demonstrate better durability when compared to organic materials.
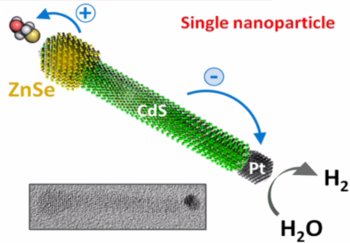 This is a schematic of the photocatalytic nanocrystal (credit: Journal of Visualized Experiments)
This is a schematic of the photocatalytic nanocrystal (credit: Journal of Visualized Experiments)
The study results have appeared in Journal of Visualized Experiments. The two nanocrystals produced by the liquid phase synthesis technique generate an electric charge or hydrogen gas during their exposure to light. Lead author, Zamkov stated that enabling direct, all inorganic coupling of the catalyst and the light absorber is the key feature of this technique.
Zamkov's nanocrystals are inorganic, have high durability, and separate charges in unique ways because of their architectures. The first nanocrystal has a rod shape and enables the charge separation required for hydrogen gas production. This reaction is called photocatalysis.
The second nanocrystal is made of stacked layers and produces electricity, therefore being a photovoltaic material. Since the nanocrystals are inrogranic materials, they can be recharged more easily and have lower heat sensitivity when compared to their organic counterparts. Zamkov's inorganic photocatalytic material facilitates a rechargeable reaction during its exposure to cheap organic solvents, while the catalyst is usually irreversibly degraded in conventional photocatalytic reactions. The photovoltaic nanocrystal is capable of withstanding higher heat when compared to conventional photovoltaic cells, which have inferior heat-dissipating properties.
Zamkov stated that the researchers developed an innovative technique to synthesize photocatalytic and photovoltaic materials, providing a new strategy to fabricate 100% inorganic photovoltaic films, which in turn paves way to manufacture high-stability solar panels.