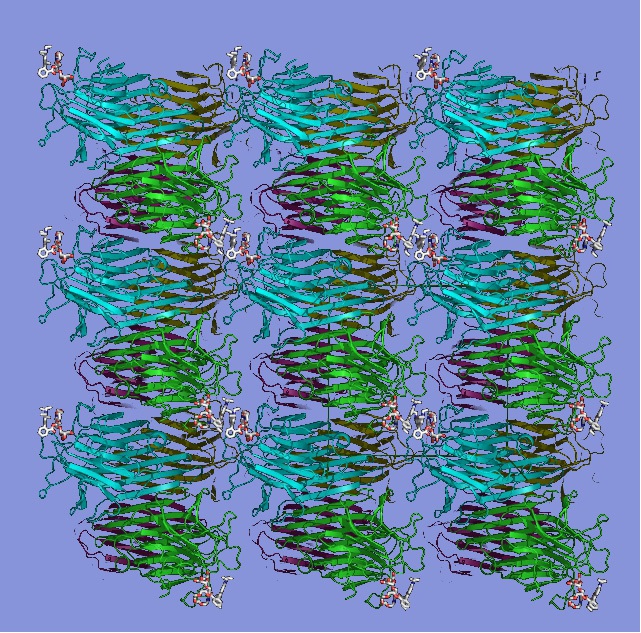
 Arrangement of protein concanavalin A molecules in a protein crystalline framework. Credit: Fudan University/HZB
Arrangement of protein concanavalin A molecules in a protein crystalline framework. Credit: Fudan University/HZB
A new class of materials, known as protein crystalline frameworks (PCFs), have been discovered by a team of researchers from Helmholtz Center Berlin (HZB) and the Fudan University in China.
PCFs proteins can form highly stable crystals by aligning symmetrically due to the use of helper substances. Although protein crystals are very delicate, sensitive and difficult to handle, scientists have been able to crystallise proteins in their native state. This has allowed researchers to examine the structure of the proteins at high resolution.
Scientists at the Fudan University have succeeded in linking concanavalin A protein to helper molecules from the sugar family and to the dye rhodamin. These fixated concanavalin molecules symmetrically arrange themselves within the framework of the helper molecules, which leads to a crystal formation in which the proteins are finely interconnected. These structures have high stability as a result of the protein crystalline framework.
In order to expand upon their research, Fudan University scientists sought the help of HZB. A Chinese scientist at HZB suggested using the HZB-MX beamlines at BESSY II electron storage ring in order to view the structure and the formation of the PCFs at the atomic level.
The two research groups collaborated and performed the high resolution characterisation of PCFs at HZB's specialized crystallography stations.
The use of helper molecules enabled the scientists to alter the power at which they penetrated the protein frameworks, provided variability and flexibility to the PCFs, which will be important in the future for further studies into potential applications.
The researchers have published their findings in Nature Communications journal.