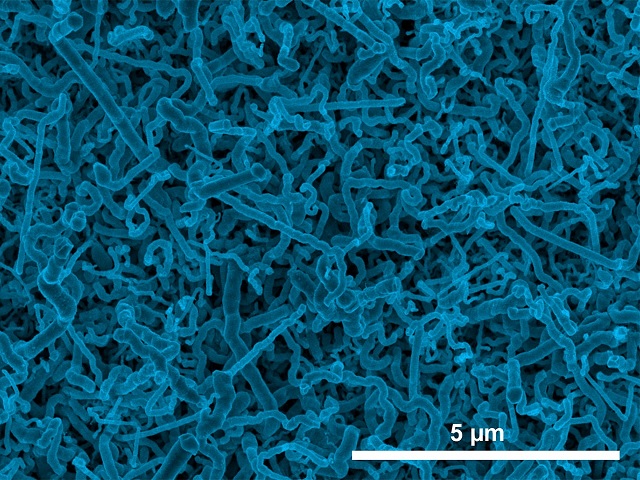
 Scanning electron micrograph image of germanium nanowires electrodeposited onto an indium-tin oxide electrode from an aqueous solution. (Jay A. Switzer/Missouri University of Science and Technology)
Scanning electron micrograph image of germanium nanowires electrodeposited onto an indium-tin oxide electrode from an aqueous solution. (Jay A. Switzer/Missouri University of Science and Technology)
A simple, low-cost, single-step approach to grow germanium nanowires from an aqueous solution has been developed by Missouri University of Science and Technology researchers, paving the way to construct improved lithium-ion batteries.
The high manufacturing cost of germanium prevents its widespread adoption in applications, including transistors, solar cells and batteries. The research group led Dr. Jay A. Switzer at Missouri S&T successfully grew nanowires of other materials using an electrodeposition process. However, the team found it difficult to grow germanium at the nano-scale and therefore used a different approach.
The research group modified the electrochemical liquid-liquid-solid process (ec-LLS), an electrodeposition process designed by a group of researchers at the University of Michigan, in order to grow nanowires of germanium using liquid metal electrodes. In the ec-LLS process, the liquid metal serves as an electrode to aid the electrodeposition and as a solvent for recrystallization of nanoparticles.
Using this process, the Missouri S&T team formed indium nanoparticles in a solution consisting of germanium dioxide, or Ge(IV) through electrochemical reduction of indium-tin oxide (ITO). The indium nanoparticle linked with the ITO functions as the electrode for Ge(IV) reduction and the reduced Ge dissolves into the particle. Germanium starts crystallising from the nanoparticle, leading to the growth of the germanium nanowire.
The germanium nanowires were grown at room temperature and at 95˚C to test the influence of temperature on the electrodeposition process. The researchers found that the process temperature had no significant effect on the nanowire quality, though the room temperature process yielded nanowires of smaller diameters. Switzer is convinced that this single-step process at room temperature shows promise to economically grow the germanium nanowires.