A synthetic amino acid capable of influencing the 3D structure of bioactive peptides and improving their potency has been developed by researchers at EPFL.
The 3D structure of a drug molecule influences how effective it is against a disease-causing target and the level of side-effects and toxicity to the patient. The molecule can bind with the target with high specificity if it has a shape matching the target.
Most of the drugs are derived from naturally existing proteins and peptides. Proteins and peptides have a specific sequence of amino acids with different 3D structures, thus exhibiting different biological functions.
However, the demand for more-efficient drugs is ever-increasing due to diseases constantly evolving. Directed evolution is an advanced field that can handle this demand by imitating natural selection in the laboratory to create new proteins and peptides.
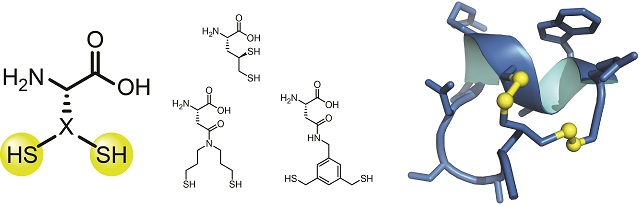
The synthetic amino acid developed by the research group at EPFL has developed a novel structure capable of significantly increasing the effectiveness of therapeutic proteins and peptides. Its structure resembles that of cysteine, a naturally occurring amino acid with a sulphur group. The presence of a sulphur group allows a bridge formation between cysteines, influencing the overall 3D structure and ultimately the biological function of a protein or peptide.
The cysteine-like amino acid is capable of forming two bridges rather than one because the single sulphur group is replaced by a branch consisting of two sulphur groups. The team synthesised five new amino acids and coupled them into the structure of cyclic peptides. The resulting peptides displayed improved biological functions.
Bicyclic peptides are a new type of therapeutic peptides that can reach disease targets that are unreachable by previously used large antibodies or small molecules. Using directed evolution, different bicyclic peptides have been developed by the EPFL team for a variety of disease targets.
The team at EPFL plans to test the new amino acid in directed evolution experiments. With structural features and the capability of creating cyclic peptides, the new synthetic amino acid holds the potential to develop of highly efficient polycyclic peptides which can be used in targeted therapy applications.