Researchers at Ohio State University have created the world's first solar battery by combining a battery and a solar cell into one hybrid device.
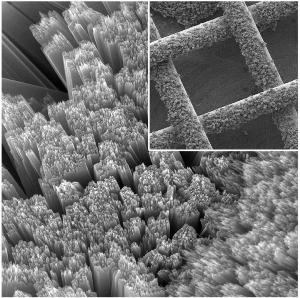 Image credit: Yiying Wu, The Ohio State University.
Image credit: Yiying Wu, The Ohio State University.
The team at Ohio State University combined a mesh solar panel, which allows air to flow into the battery, and a special process which allows electrons to flow between the battery electrode and the solar panel. The battery is charged by chemical reactions between light and oxygen.
Titanium gauze, a bendable material, was used to create the porous mesh solar panel with vertical titanium dioxide rods which were as thin as grass added to the panel.
In previously created devices four electrodes were required to connect a solar cell to a battery, whereas in the new hybrid battery only three were required. The mesh solar panel acted as the first electrode, a thin porous carbon sheet below the panel became the second electrode, and finally a lithium plate formed the third.
An iodide additive in the electrolyte is another unique feature in the device which enhances the performance and efficiency of the battery.
Within the battery, electrons aid in the chemical decomposition of lithium peroxide which transforms into oxygen and lithium ions. Oxygen is then let out while the ions are stored in the battery as lithium metal. Each time the battery discharges, it chemically absorbs oxygen from the air to re-build lithium peroxide.
According to the researchers, their solar battery will help to reduce costs by at least 25%. Another key advantage is that in earlier models, only 80% of electrons from a solar cell were collected in a battery, whereas in the new solar battery design light is converted to electrons within the battery, thereby saving almost 100% of the electrons.
The study was published in the journal Nature Communications.