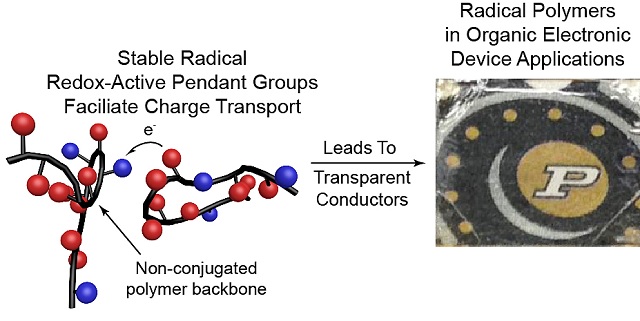
 An emerging class of electrically conductive plastics are called "radical polymers.” The graphic at left depicts the structure of a polymer. At right, transparent polymer overlays the Purdue logo. (Courtesy: Purdue University photo)
An emerging class of electrically conductive plastics are called "radical polymers.” The graphic at left depicts the structure of a polymer. At right, transparent polymer overlays the Purdue logo. (Courtesy: Purdue University photo)
Scientists from Purdue University have created a new class of electrically conductive polymers known as PTMA which are likely to revolutionise the manufacture of smart lightweight batteries, ultrathin antiglare coatings and transparent solar cells for the aircraft and electronics markets in a relatively inexpensive manner.
These newly discovered radical polymers can improve the electrical conductivity by nearly 10 times when compared to the semiconducting polymers currently used. This polymer can be easily produced and is similar to Plexiglas – however unlike Plexiglas, PTMA conducts electricity.
We make billions of tons of plastic every year. So imagine if you could produce that same kind of material at that same scale but now it has electronic properties.
Bryan Boudouris, Assistant Professor of Chemical Engineering at Purdue University
Although the polymers have already found commercial applications in the production of the next generation of batteries, finding widespread uses for PTMA will require their electrical conductivity to be improved by around another 1000 times.
PTMA was created by the Purdue scientists using a process known as deprotection, in which a particular hydrogen atom located in the pendant group is substituted with an oxygen atom, thereby transforming it into a radical group. The PTMA’s oxygen atom consists of a single unpaired electron in its outer shell, helping it to carry charge.
The scientists proved that the deprotection process could create four different chemical functionalities of PTMA, out of which two may help increase the polymer’s conductivity. The resultant chemical functionalities have to be closely observed and the reaction conditions during deprotection have to be manipulated so as to be able to fine tune the electrical properties of PTMA.
The findings from this study are detailed in the journal Macromolecules.