Oct 13 2014
Rockefeller University researchers have identified a new method which reveals the complex molecular structure of cholesterol by mapping a key enzyme structure during cholesterol production.
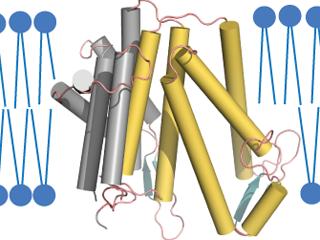 An enzyme responsible for a crucial step in the production of cholesterol has ten segments that span the cell membrane in which it is embedded. These contain two pockets the enzyme uses to bring the reactants together. Credit: Laboratory of Cell Biology at The Rockefeller University/Nature
An enzyme responsible for a crucial step in the production of cholesterol has ten segments that span the cell membrane in which it is embedded. These contain two pockets the enzyme uses to bring the reactants together. Credit: Laboratory of Cell Biology at The Rockefeller University/Nature
Cholesterol, regardless of its harmful effects, is an essential substance for normal functioning of the human body. It is naturally produced by cells in order to strengthen the cellular membrane and protect it from cellular damage.
This is the first time the position of every atom of the 3000 contained within the key enzyme has been clearly mapped. Studying the structure of the key enzyme provides a better understanding of the synthesis of cholesterol in the body.
The research team believe that this technique could pave the way for the development of new approaches to treat genetic disorders and high cholesterol levels.
Cholesterol, a precursor to specific hormones such as bile, vitamin D and testosterone, is produced within the cells by 30 chemical reactions, using 20 enzymes out of which seven are membrane embedded.
The mapping is based on the reduction process of one of these enzymes called sterol reductase. This enzyme helps in producing cholesterol by transferring two electrons from NADPH to another molecule.
The researchers said that the image obtained after mapping displayed two pockets within the enzyme’s structure – one with the NADPH while the other providing the way to cholesterol precursor.
Initially, the key molecule of interest in this research is the lamin B receptor (LBR) which resulted in poor crystallization. Therefore, they used the maSR1 protein from a methane eating bacterium which was proved to perform the same function as LBR.
The X-ray diffraction studies of maSR1 crystals showed a protein consisting 10 parts in which half of the molecules carried two pockets for mixing the reactants. The researchers believe that the other half is used for the interaction with enzymes producing cholesterol.
In addition, the research team identified the region of defects in the molecule in order to understand the phenomenon of enzyme alteration due to mutation. Mutations result in various disorders such as Pelger-Huet Anomaly and Smith-Lemli-Opitz. The technique can also be used to treat high levels of cholesterol in the body.