Researchers from the Purdue University stumbled upon a novel technique that uses a pulsing laser to produce synthetic nanodiamond patterns and films from graphite, while trying to analyze a technique to strengthen metals. Their discovery is likely to impact a wide range of applications such as biosensors, computer chips, quantum computing, and fuel cells.
 This illustration depicts a new technique that uses a pulsing laser to create synthetic nanodiamond films and patterns from graphite, with potential applications from biosensors to computer chips. (Courtesy: Purdue University image/Gary Cheng)
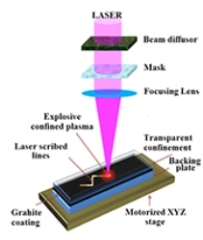
This illustration depicts a new technique that uses a pulsing laser to create synthetic nanodiamond films and patterns from graphite, with potential applications from biosensors to computer chips. (Courtesy: Purdue University image/Gary Cheng)
While trying to increase the strength of metals using a thin graphite sheet and a nanosecond-pulsing laser, the researchers observed that using the laser made the graphite to either disappear or become semi-transparent. They realized that the graphite’s black coating disappeared. By conducting follow up research, they established that the graphite was transformed into diamond. They termed the process as confined pulse laser deposition (CPLD).
One of the researchers explained that the nanodiamond could be selectively placed on rough surfaces without the need for high pressures and temperatures, which are generally associated with the production of synthetic diamond. They were able to perform CPLD at room temperature, thus greatly reducing the cost of producing diamond.
The novel technique requires the use of a multilayered film comprising a layer of graphite topped with a glass cover sheet. This layer is then exposed to an ultrafast-pulsing laser, which acts on the graphite to change it into ionized plasma and generates a downward pressure. The ionized plasma soon becomes a solidified diamond. The purpose of the glass cover sheet is to keep the plasma in place, thereby enabling it to develop a nanodiamond coating.
The research team proved that the diamond created using their technique was real using conventional methods such as the X-ray diffraction, transmission electron microscopy, and the measurement of electrical resistance.
These are super-small diamonds and the coating is super-strong, so it could be used for high-temperature sensors.
Gary Cheng, an associate professor of industrial engineering at Purdue University.
References