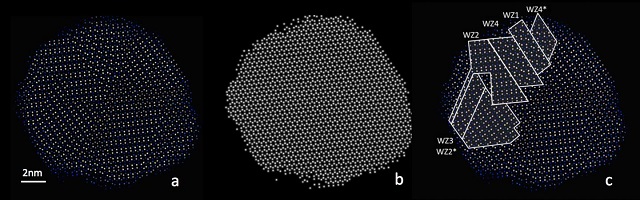
 These are three atomic-resolution views of a copper-indium-sulfur nanoparticle: (a) Only the copper and indium atoms are shown in blue and yellow; (b) Copper and indium atoms are shown in same color demonstrating that they form a perfect hexagonal lattice; (c) Same as (a) with the boundaries between regions of distinct copper-indium order outlined. Credit: Xiao Shen, Vanderbilt University
These are three atomic-resolution views of a copper-indium-sulfur nanoparticle: (a) Only the copper and indium atoms are shown in blue and yellow; (b) Copper and indium atoms are shown in same color demonstrating that they form a perfect hexagonal lattice; (c) Same as (a) with the boundaries between regions of distinct copper-indium order outlined. Credit: Xiao Shen, Vanderbilt University
Researchers have discovered an innovative form of crystalline structure that displays crystal and polycrystalline properties at the same time. The research was a joint effort between Oak Ridge National Laboratory (ORNL) and Vanderbilt University.
Dubbed as "interlaced crystals", the unique crystal arrangement of atoms was discovered by researchers while they were exploring semiconductor copper-indium sulfide or CIS-based nanoparticles. The study has been published in the journal Nature Communications.
The new form of crystalline order exhibits properties, which make it suitable for thermoelectric applications. Materials that have enhanced thermoelectric efficiency can possibly increase automobile mileage, boost the efficiency of power generation, and cut down the cost of air conditioning.
Atoms in crystalline materials are organized in periodic arrays of points known as Bravais lattice. Each lattice point is occupied by the same atom or a set of atoms. A typical example of two-dimensional structure is the square floor tile, while a three-dimensional example is the face centered cubic (FCC), which exhibits points not only at the corners but also at the middle faces of the cube.
In CIS, one FCC sub-lattice is occupied by the sulfur atoms, while the second sub-lattice is occupied by the indium and copper atoms. Each sulfur atom is enclosed by two adjacent indium and two copper atoms, while each indium or copper atom is enclosed by four neighboring sulfur atoms. Although bulk CIS typically has a cubic structure, it was found that the tiny crystals also feature a hexagonal lattice structure. Due to the tiny size of nanoparticles, the ordered structure of the indium and copper atoms could not be distinguished by X-ray diffraction.
The researchers later captured detailed images of nanoparticles, which showed that all atoms reside at the points of a hexagonal Bravais lattice, while the indium and copper atoms formed an array of domains where the indium and copper atoms were organized in a different fashion. The images also showed that the underlying hexagonal lattice was not disturbed. In polycrystalline samples, strain will be present at the edges of different areas, but in CIS there were no signs of breaks or strains at the edges. This interlaced crystal structure could help improve thermoelectric applications for cooling or power generation. Thermoelectric instruments require materials that are poor heat conductors, but excellent electrical conductors. However, metals that are good conductors of heat also tend to be good conductors of electricity and vice versa.
We haven't tested this yet, but we are confident that these materials have high electrical conductivity and low thermal conductivity...just what you need for thermoelectrics. The field is now wide open for scientists who can fabricate thin films and make thermoelectric measurements.
Sokrates Pantelides, University Distinguished Professor of Physics and Engineering at Vanderbilt University
References