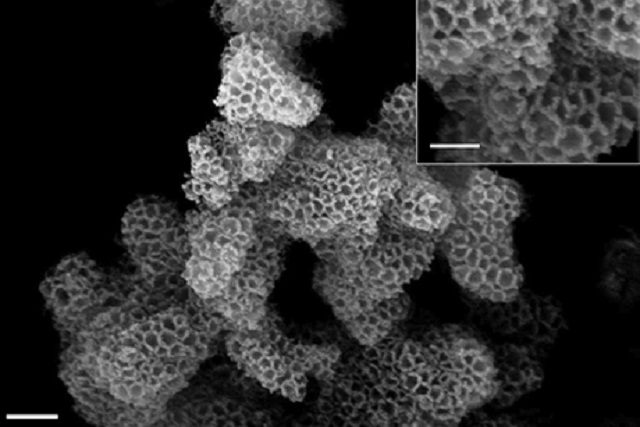
 A scanning electron microscopy image of a pristine silica support, before the amine is added. Credit: Provided/Genggeng Qi
A scanning electron microscopy image of a pristine silica support, before the amine is added. Credit: Provided/Genggeng Qi
Materials scientists at Cornell University have developed highly effective “sponges” that can trap carbon and help cut down on greenhouse gases. Carbon capture is gaining momentum in the world’s fight against global warming. Carbon capture involves trapping carbon dioxide chemically before it gets released to the atmosphere. However, most methods are inefficient, corrosive and toxic.
Emmanuel Giannelis, the Walter R. Read Professor of Engineering in the Department of Materials Science and Engineering at Cornell University, led the research team. They have developed a powder that performs better than the standards set by the industry for carbon capture. Genggeng Qi and Liling Fu are postdoctoral associates who have co-authored a paper on this study.
Presently, amine scrubbing is the most widely used method of carbon capture. This is used in coal and natural gas burning plants. In this method, after combustion, the flue gas that contains carbon dioxide is made to go through liquid amino compound vats. Here, most of the carbon dioxide gets absorbed. The resulting carbon-rich gas is reused or sequestered. Furthermore, the amine solution becomes highly corrosive and its containment requires significant cost
A scanning electron microscopy image of a pristine silica support, before the amine is added.
From 2008, researchers have been trying to develop safer and better carbon-capture methods, and numerous methods have been developed. The latest carbon-capture method involves a silica scaffold having nanoscale pores for ensuring maximum surface area. Here, the silica scaffold is the sorbent support. The scaffold is dipped into the liquid amine. This liquid soaks into the sorbent support in the same way as a sponge functions. It then hardens partially. The final product is a dry, stable white powder that has the ability to capture carbon dioxide even when moisture exists.
Giannelis stated that solid amine sorbents were used in carbon capture; however amines are impregnated physically into the support. Some of the amine gets lost over a period of time, which lowers the effectiveness of the sorbents. This leads to increased cost. In the Cornell study, the amine was grown onto the sorbent surface. This led to a chemical bond between the amine and the sorbents, which reduced the loss of amine over a period of time.
Qi stated that the researchers now aim to optimize the sorbent which would allow it to be demonstrated for the industry. This could be done at Cornell’s power plant by retrofitting the equipment. The same technology could be applied on a smaller scale to enhance plant growth using carbon dioxide captured in greenhouses.
Earlier this year, Giannelis had presented on this topic to Cornell’s vice president for facilities services, KyuJung Whang.
We have made great strides in sustainability, particularly in the energy supply areas of alternative energy sources, and the demand side areas of energy conservation and building design standards. If we are truly to achieve neutrality, though, we also have to consider capturing and offsetting carbon. Emmanuel’s presentation got my attention, and I was hoping to learn more about it and explore ways we might be able to work together.
KyuJung Whang
The King Abdullah University of Science and Technology (KAUST) and Qatar University have supported this study. The National Science Foundation has funded the Cornell Center for Materials Research which was used for this study. The KAUST-Cornell Center for Energy and Sustainability was used for performed scaling-up experiments.
This study titled “Sponges with Covalently Tethered Amines for High-Efficiency Carbon Capture” has been published in Nature Communications journal.
References