Schematic of the preparation of a 3-D hierarchically structured graphene-sulfur/carbonZIF8-D composite.
Image credit: K.Xi / Cambridge
A team of researchers from the UK and China have designed a sulfur cathode wrapped with graphene for use in rechargeable lithium-sulfur batteries.
Dr. Vasant Kumar from the University of Cambridge and Professor Renjie Chen from the Beijing Institute of Technology led the research team for this project.
The researchers managed to solve low efficiency, capacity degradation and other performance-related issues at the nanolevel. A potentially ground breaking design for the electrode structure in rechargeable lithium-sulfur batteries is created when a thin sheet of graphene is wrapped around an innovative multifunctional sulfur electrode. This comprises an electron/ion transfer network and an energy storage unit.
Theoretically, lithium-sulfur batteries possess greater energy densities than lithium ion batteries. This has unsurprisingly led to considerable commercial interest.
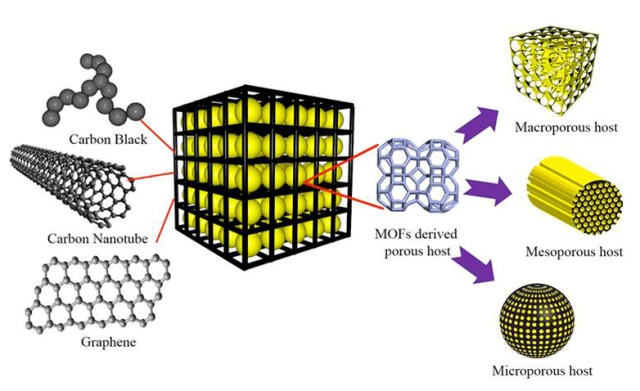
Metal organic frameworks (MOFs) are used in a wide range of applications including hydrogen storage, catalysis, membranes and carbon dioxide sequestration, and their properties are rather unique.
The research team used an MOF as a template in order to create a conductive porous carbon cage. In this cage, the sulfur acts as the host. When electrochemical reactions take place, every sulfur-carbon nanoparticle acts as an energy storage unit.
Our carbon scaffold acts as a physical barrier to confine the active materials within its porous structure. This leads to improved cycling stability and high efficiency.
Kai Xi
Researcher at the University of Cambridge
The researchers also found that when the sulfur-carbon energy storage unit was again wrapped within a thin flexible graphene sheet it sped up the ion and electron transport.
The researchers state that the interconnected graphene network possessing high electrical conductivity enabled rapid charge-transfer kinetics. This study demonstrates that a porous scaffold’s composite structure, having conductive connections, holds promise for the design of the electrode structure of rechargeable batteries.
This work provides a "basic, but flexible, approach to both enhance the use of sulfur and improve the cycle stability of batteries," Xi said. "Modification of the unit or its framework by doping or polymer coating could take the performance to a whole new level."
The innovative battery integrates an ion/electron framework and energy storage unit in a unique manner. This allows it to be used for fabricating high-performance energy storage systems that are non-topotactic and reaction-based. In non-topotactic reactions crystalline solids do not undergo structural changes.
Speaking about future projects:
We'll focus on fabricating hybrid free-standing sulfur cathode systems to achieve high-energy density batteries, which will involve tailoring novel electrolyte components and building lithium 'protection layers' to enhance the electrochemical performance of batteries.
Kai Xi
Researcher at the University of Cambridge
This study has been published in the journal, APL Materials, from AIP Publishing.
Lithium-ion batteries: How do they work? - BASF YouTube
This News Feature Around the Web