Feb 25 2015
Researchers at the Aalto University in Finland have developed an efficient and cost-effective substitute for platinum in energy storage applications.
Platinum is an expensive and rare metal. Electrolysers are designed to store electric energy in the form of chemical compounds, and platinum has been used extensively as an electrocatalyst in these electrolysers.
However, the increasing use of renewable energy poses a challenge, due to the extra capacity required to store the electricity produced.
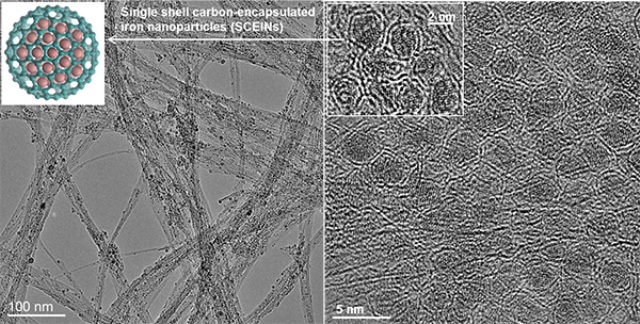
Now, Aalto University researchers have created an electrocatalyst made of carbon nanotubes and iron nanoparticles, which could store large amounts of electricity more cost-effectively.
We developed an electrocatalyst that is made of iron and carbon. Now the same efficiency that was achieved with platinum can be obtained with a less expensive material. Nearly 40 per cent of the material costs of energy storage with an electrolyser come from the electrocatalyst
Tanja Kallio, senior scientist
Professor Esko Kauppinen from the Aalto University School of Science led a research group that developed a single-stage manufacturing process for the catalyst material.
The material consists of carbon nanotubes, which act as a support, and also have very high electrical conductivity. Iron nanoparticles encapsulated in a single-layer carbon shell act as the active catalyst.
The method has been altered to make the electro catalyst very active. By active, we refer to the small amount of energy needed to store electric energy as hydrogen. This reduces the losses caused by chemical storage and the process is economically viable.
Professor Kari Laasonen and Senior scientist Tanja Kallio led research groups who had conducted this study at the Aalto University School of Chemical Technology. The Aalto University’s Aalto Energy Efficiency (AEF) Research Programme had provided funds for this study.
The researchers have published their study in the journal Angewandte Chemie.
Sources and Further Reading