Jun 17 2015
An international research team has found a new method of protecting sensitive catalysts from damage caused by oxygen, with the help of a hydrogel. The researchers from Bochum and Mülheim carried out this experiment along with French colleagues, and stated that the hydrogel acts as a protective shield for biomolecules and could potentially result in hydrogen fuel cells that have molecular catalysts or biomolecules such as the hydrogenase enzyme. Until now, this was possible using only the expensive and rare precious metal platinum.
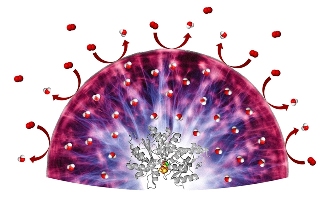 With a novel hydrogel, sensitive catalysts can be protected from oxygen molecules (red) which could irreversibly damage the catalysts. The hydrogel converts oxygen into water (red-white).
With a novel hydrogel, sensitive catalysts can be protected from oxygen molecules (red) which could irreversibly damage the catalysts. The hydrogel converts oxygen into water (red-white).
Requirements on catalysts are difficult to reconcile
Most industrial applications demand affordable, stable and efficient catalysts. Additionally, they need to be customized for a particular chemical reaction. Dr Nicolas Plumeré from the Chemistry Department at the Ruhr-Universität Bochum said, “Uniting all of these requirements in one molecule is a considerable challenge.” Using a versatile hydrogel embedded with catalysts could greatly simplify fuel cell catalyst development in the future. The researchers from Bochum started a project along with their colleagues from the Max Planck Institute for Chemical Energy Conversion in Mülheim and from Aix Marseille University and the Centre National de la Recherche Scientifique (CNRS) in France to investigate this new possibility.
Hydrogel acting as solvent and as protecting environment
The German team used the green alga Chlamydomonas rheinhardtii to extract the hydrogenase enzyme for their experiments. The enzyme divides the hydrogen into electrons and protons. The biomolecule can be damaged irreversibly in contact with even a small trace of oxygen. The researchers, however, incorporated it into the hydrogel for two reasons. Firstly, it serves as a solvent, ensuring that all the reaction partners easily and quickly reach the enzyme, and secondly, it offers a protective environment which prevents penetration of oxygen into the enzyme, even if it is present at relatively high concentrations. Typically, the electrons are created as a result of hydrogenase activity. The electrons wander across the hydrogel, and are transmitted to the oxygen, thereby transforming it into a harmless substance i.e. water.
Catalyst design could become considerably easier in the future
The German-French team explained another key aspect of hydrogels using simulations and experiments. The exposure to deactivating molecules suppresses the activity of different catalysts. However, some of them can be made functional via special reactivation processes. The hydrogel was observed to protect even the catalysts which do not require a reactivation process.
“In future, we will thus no longer have to pay attention to the robustness or suitable reactivation processes when developing catalysts for technical applications. We can focus solely on maximising the catalyst’s activity. This will simplify the development process to a considerable degree and open up new possibilities for the manufacture of fuel cells,” explained Olaf Rüdiger, Chemist at the Max Planck Institute for Chemical Energy Conversion.
The research, published in the journals “Angewandte Chemie” and “The Journal of the American Chemical Society” was funded by the German Research Foundation as part of the RESOLV Cluster of Excellence (EXC 1069).