Researchers at MIT and other institutes have identified that two processes previously thought to be unrelated, catalysis and wetting, are closely related to each other. This surprising result could help to identify new catalysts for specific applications.
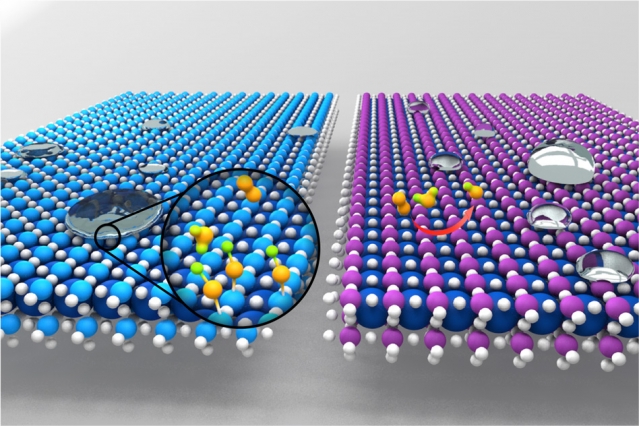 Materials that have good wetting properties, as illustrated on the left, where droplets spread out flat, tend to have hydroxyl groups attached to the surface, which inhibits catalytic activity. Materials that repel water, as shown at right, where droplets form sharp, steep boundaries, are more conducive to catalytic activity, as shown by the reactions among small orange molecules. (Credit: Xiao Renshaw Wan)
Materials that have good wetting properties, as illustrated on the left, where droplets spread out flat, tend to have hydroxyl groups attached to the surface, which inhibits catalytic activity. Materials that repel water, as shown at right, where droplets form sharp, steep boundaries, are more conducive to catalytic activity, as shown by the reactions among small orange molecules. (Credit: Xiao Renshaw Wan)
Both catalysis and wetting occur at a material's surface. A catalyst is used to improve the chemical reaction rate, whereas the wetting process supports the spreading of liquid across the surface.
The research, published in the Journal of Physical Chemistry C, is mainly focused on the oxide group known as perovskites that are widely employed in many applications, such as fuel cells, batteries, water purification and gas sensing.
“What’s really exciting is that we’ve been able to connect atomic-level interactions of water and oxides on the surface to macroscopic measurements of wetting, whether a surface is hydrophobic or hydrophilic, and connect that directly with catalytic properties,” says Yang Shao-Horn, the W.M. Keck Professor of Energy at MIT and a senior author of a paper.
According to senior author Kripa Varanasi, an associate professor of mechanical engineering, a material’s suitability as a catalyst can be easily identified by determining the surface’s wettability, which is “trivially easy.” He added that since scientists want to become an expert in either wettability or catalysis, this provides a framework for scientists in both areas to work collaboratively for better understanding. Varanasi's research is mainly focused on wettability, whereas Shao-Horn is working on catalytic reactions.
We show how wetting and catalysis, which are both surface phenomena, are related and how electronic structure forms a link between both.
Although both processes are critical to a number of industrial processes and are of major interest in empirical research, “at the molecular level, we understand very little about what’s happening at the interface,” Shao-Horn says. “This is a step forward, providing a molecular-level understanding.”
According to Kelsey Stoerzinger, an MIT graduate student and the paper’s lead author, it was “primarily an experimental technique” that led to the new understanding. Unlike most of the research carried out to study this surface science using vacuum-based instruments, this team deployed a device capable of investigating the reactions in humid air at room temperature, and with different levels of water vapor present. Based on the experiments performed using the system known as ambient pressure X-ray photoelectron spectroscopy, it is evident that the reactivity with water is important for the entire process.
Hydroxyl groups, consisting of an oxygen atom bound to a hydrogen atom, are formed on the surface of the material when water molecules break down. These reactive compounds are the main reason for increasing the surface's wetting properties, while suppressing the catalysis of chemical reactions in the surface. Hence, the researchers found that hydrophobic/non-wetting surface is the main requirement for applications that demand high catalytic activity.
“Ideally, this understanding helps us design new catalysts,” Stoerzinger says. She commented that if a given material “has a lower affinity for water, it has a higher affinity for catalytic activity.”
Shao-Horn notes that this is an initial finding, and that “extension of these trends to broader classes of materials and ranges of hydroxyl affinity requires further investigation.” The team has already begun working on these areas. This research, she says, “opens up the space of materials and surfaces we might think about” for both catalysis and wetting.
Other members of the research team include graduate student Wesley Hong, visiting scientist Livia Giordano, postdocs Yueh-Lin Lee and Gisele Azimi at MIT, Ethan Crumlin and Hendrik Bluhm at Lawrence Berkeley National Laboratory, and Michael Biegalski at Oak Ridge National Laboratory.
The National Science Foundation and the U.S. Department of Energy supported this research.