Researchers have developed a new and versatile method to carry out light-driven reactions, paving the way for another energy-generation technology by harnessing sunlight. This method is based on plasmon, a special type of electron movement that influences the optical properties of metals.
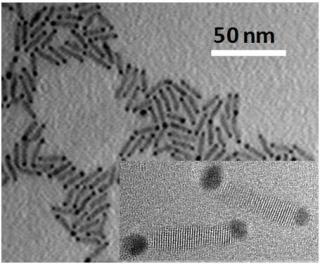 This is a transmission electron micrograph image of cadmium selenide nanorods with gold tips. Inset shows a high-resolution TEM image of two nanorods. (Credit. Micrograph images by James R. McBride)
This is a transmission electron micrograph image of cadmium selenide nanorods with gold tips. Inset shows a high-resolution TEM image of two nanorods. (Credit. Micrograph images by James R. McBride)
"We've discovered a new and unexpected way to use plasmonic metal that holds potential for use in solar energy conversion," says Tim Lian, professor of physical chemistry at Emory University and the lead author of the research. "We've shown that we can harvest the high energy electrons excited by light in plasmon and then use this energy to do chemistry."
Plasmon is a collective movement of free electrons in a metal with strong light absorbing and scattering capabilities. One of the most interesting surface plasmon examples can be observed in the finely stained glass windows of certain medieval cathedrals, which are achieved using gold nano-particles with visible light absorption and scattering capabilities. Plasmon can be easily tuned: the color of the emitted light can be controlled by changing the shape and size of the gold nano-particles in the glass.
The use of such plasmonic effects for applications ranging from electronics to medicine and renewable energy is being explored and improved through advanced technology.
Researchers in Lian's lab, which involves research related to light-driven charge transfer for solar energy conversion, has demonstrated different methods of using plasmon to achieve a more versatile and sustainable process.
Gold is predominantly used as a catalyst, which is a substance used for driving chemical reactions, and not as a photo-catalyst which absorbs light and carries out chemical reactions using the energy provided by the light.
The light is strongly absorbed by a metal during photocatalysis, thereby exciting a number of electrons. "Imagine electrons sloshing up and down in the metal," Lian says. "Once you excite them at this level, they crash right down. All the energy is released as heat really fast - in picoseconds."
The researchers focused on identifying a means of capturing the energy present in the excited electrons prior to its release in the form of heat, and used hot electrons to drive reactions.
It was evident from the findings that the excited electrons present in the gold were made to escape into the semiconductor material upon combining the nano-rods of cadmium selenide semi-conductor with a plasmonic gold nanoparticle tip.
"If you use a material with a certain energy level that can strongly bond to plasmon, then the excited electrons can escape into the material and stay at the high energy level," Lian says. "We showed that you can harvest electrons before they crash down and relax, and combine the catalytic property of plasmon with its light absorbing ability."
This new method makes use of light and metals to carry out photochemistry rather than using heat, thereby paving the way for a new, efficient method to explore.
"We are now looking at whether we can find other electron acceptors that would work in this same process, such as a molecule or molecular catalyst instead of cadmium selenide," Lian says. "That would make this process a general scheme with many different potential applications."
The researchers are also interested in determining if this method can trigger light-based water oxidation effectively. A main objective in the quest for inexpensive and sustainable solar energy is to use sunlight to divide water and produce hydrogen.
"Using unlimited sunlight to move electrons around and tap catalytic power is a difficult challenge, but we have to find ways to do this," Lian says. "We have no choice. Solar power is the only energy source that can sustain the growing human population without catastrophic environmental impact."