A team of researchers from MIT and Samsung, along with others from California and Maryland, have developed a solid electrolyte which could vastly improve battery lifetime and safety.
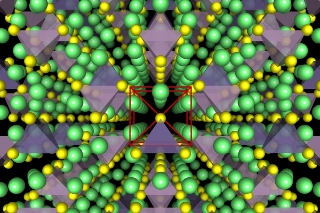 Illustrations show the crystal structure of a superionic conductor. The backbone of the material is a body-centred cubic-like arrangement of sulphur anions. Lithium atoms are depicted in green, sulfur atoms in yellow, PS4 tetrahedra in purple, and GeS4 tetrahedra in blue. Researchers have revealed the fundamental relationship between anion packing and ionic transport in fast lithium-conducting materials. Credit: Yan Wang
Illustrations show the crystal structure of a superionic conductor. The backbone of the material is a body-centred cubic-like arrangement of sulphur anions. Lithium atoms are depicted in green, sulfur atoms in yellow, PS4 tetrahedra in purple, and GeS4 tetrahedra in blue. Researchers have revealed the fundamental relationship between anion packing and ionic transport in fast lithium-conducting materials. Credit: Yan Wang
Their research found that a solid electrolyte would be more suitable than the liquid type that is currently in use in most rechargeable batteries. Apart from increasing the quantity of power stored in a specified space, the solid electrolyte would significantly enhance not just the lifetime of the device, but also the safety levels.
The research has been published in the journal 'Nature Materials', in a paper by MIT postdoc Yan Wang, visiting professor of Materials Science and Engineering Gerbrand Ceder, and five others.
Most batteries found in high-tech gadgets such as cellphones, electric cars or laptops tend to occupy a great deal of space. However, consumers are forever seeking more from their gadgets. They want the battery to last longer and their gadgets to operate longer.
There has also been vast media coverage on lithium-ion batteries overheating or even combusting, thereby raising a safety issue in battery technology. These demands have pushed researchers to find a way to increase the power that a battery of a specific size can hold. The joint partnership team illustrates that the creation of solid-state electrolytes is crucial to solving consumers’ demands and the issues relating to enhancing lithium-ion batteries.
Ceder explains that the basic liquid organic solvent electrolyte in batteries, meant for transporting charged particles from one electrode of the battery to the other, has been pinned as responsible for the overheating and fire incidents, including the incident where all of Boeing's 787 Dreamliner jets had to be temporarily grounded.
Although other researchers have tried to find a solid substitute for the liquid electrolyte, the joint partnership research team has been the first to prove that it can performed in a formulation which meets the requirement of battery applications.
Ceder states that solid-state electrolytes could be “a real game-changer,” creating “almost a perfect battery, solving most of the remaining issues” in battery safety, lifetime and cost. He adds: “All of the fires you’ve seen, with Boeing, Tesla, and others, they are all electrolyte fires. The lithium itself is not flammable in the state it’s in these batteries. [With a solid electrolyte] there’s no safety problem — you could throw it against the wall, drive a nail through it — there’s nothing there to burn.”
Cedar explains further that the planned solid electrolyte has additional benefits. He says: “With a solid-state electrolyte, there’s virtually no degradation reactions left” — meaning such batteries could last through “hundreds of thousands of cycles.”
Finding the ideal solid materials, which could rapidly conduct ions to render them useful in a battery, was the key for ensuring the feasibility of batteries that could last through so many cycles.
“There was a view that solids cannot conduct fast enough,” he says. “That paradigm has been overthrown.”
Ceder and team closely studied the factors key to creating efficient ion conduction in solids. They were able to pinpoint certain compounds possessing the right features. The preliminary results were based on a group of materials called superionic lithium-ion conductors which are compounds of lithium, phosphorus, germanium and sulfur. The team believes that their research will pave the way for advanced effective materials.
Ceder says that this research was made possible because of an ongoing partnership with the Korean electronics company Samsung, via the Samsung Advanced Institute of Technology in Cambridge, Massachusetts. This partnership also led to other advancements, such as the application of quantum-dot materials to develop very efficient sodium batteries and solar cells.
Ceder states that the solid-state electrolyte possesses other allied advantages. The traditional lithium-ion batteries do not function well in very cold environments and require pre-heating if at temperatures below around minus 20°F, whereas the solid-electrolyte model can function well in similar environments.
Furthermore, the solid-state electrolyte enables higher power density, which basically refers to the quantity of power that can be stored in a specific space. These batteries are likely to provide an increase of 20-30% in power density, resulting in a corresponding rise in the time that a specific size battery can power a computer, phone or car.
The team also included MIT graduate student William Richards and postdoc Jae Chul Kim; Shyue Ping Ong at the University of California at San Diego; Yifei Mo at the University of Maryland; and Lincoln Miara at Samsung. The work is part of an alliance between MIT and the Samsung Advanced Institute of Technology focusing on the development of materials for clean energy.