A process has been developed to produce supercapacitors from old tires. Material from the tires is converted into flexible polymer carbon composite films that can be used as electrodes in supercapacitors.
Researchers at the Department of Energy’s (DOE) Oak Ridge National Laboratory (ORNL) and Drexel University are responsible for this breakthrough in green supercapacitor production. Supercapacitors are widely used in electric grids and in vehicles. The supercapacitors with electrodes created from tires drop just by 2% even after 10,000 charge/discharge cycles.
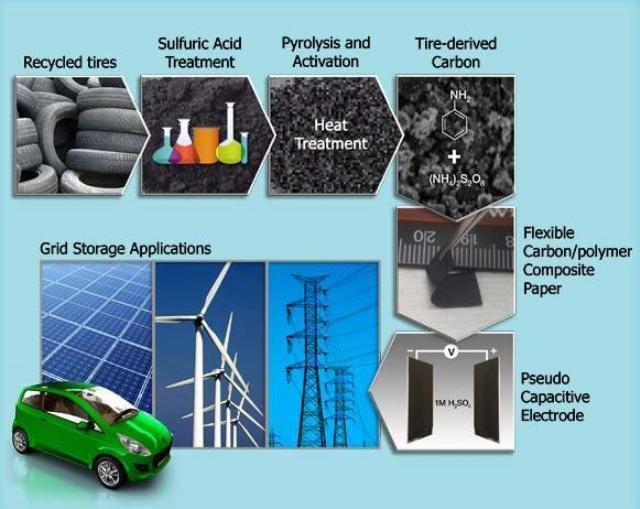 Instead of ending up in landfills, old tires be used in the production of supercapacitors to help power the nation.
Instead of ending up in landfills, old tires be used in the production of supercapacitors to help power the nation.
This innovative technology is based on a method discovered at ORNL that utilizes scrap tires for creating batteries. It is hoped that this finding will help address the huge amount of tires being discarded every year. In the United States alone, around 300 million tires are discarded annually, and by 2035, the annual production of tires is expected to be 1.5 billion tires.
Parans Paranthaman had led the research team that developed processing methods for creating the films for the electrodes.
Those tires will eventually need to be discarded, and our supercapacitor applications can consume several tons of this waste. Combined with the technology we’ve licensed to two companies to convert scrap tires into carbon powders for batteries, we estimate consuming about 50 tons per day.
- Panans Paranthaman, Oak Ridge
Every day, 8,000 tons of tires have to be recycled, and this technology helps address just a fraction of that total. Yury Gogotsi of Drexel, co-author of the study, states that the huge volume could be addressed with the contribution of other recycling companies.
Each tire can produce carbon with a yield of about 50 percent with the ORNL process. If we were to recycle all of the scrap tires, that would translate into 1.5 million tons of carbon, which is half of the annual global production of graphite.
- Yury Gogotsi, Drexel
The team soaked irregularly shaped tire rubber in the form of crumbs in concentrated sulfuric acid. This rubber was then washed, and loaded into a tubular furnace under an atmosphere of flowing nitrogen gas. The furnace temperature was then increased gradually from 400°C to 1100°C. The material was then mixed with potassium hydroxide, baked, washed with deionized water, and oven dried. The resulting material had the capability to mix with an electrically conductive polymer called a polyaniline.
We anticipate that the same strategy can be applied to deposit other pseudocapacitive materials with low-cost tire-derived activated carbon to achieve even higher electrochemical performance and longer cycle life, a key challenge for electrochemically active polymers
- Yury Gogotsi, Drexel
The study paper, entitled, “Waste Tire Derived Carbon–Polymer Composite Paper as Pseudocapacitive Electrode with Long Cycle Life,” has been published in ChemSusChem.
Other authors who contributed to the study include Muhammad Boota of Drexel University; and Amit Naskar, Kokouvi Akato and Kokouvi Akato, all of ORNL.
The DOE’s Office of Science and the Laboratory Directed Research and Development and Technology Innovation programs at ORNL provided funding for this study.