Oct 13 2015
A team of researchers from Tufts University has discovered that an advanced type of platinum-copper catalyst needs only a limited concentration of platinum, in the form of individual atoms, to execute vital chemical reactions in a cost-effective and clean manner.
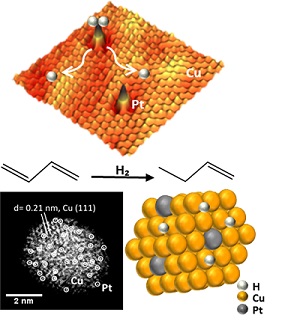 Atomic-resolution microscopy shows the single platinum atoms on copper nanoparticles that can split hydrogen into atoms enabling the efficient and selective hydrogenation of butadiene.
Atomic-resolution microscopy shows the single platinum atoms on copper nanoparticles that can split hydrogen into atoms enabling the efficient and selective hydrogenation of butadiene.
Due to its outstanding capability to assist numerous chemical reactions, platinum is used as a catalyst in automobile converters, in fuel cells, and in the chemical industry. The use of platinum in a wider scale is not possible because of its cost and scarcity.
Furthermore, platinum readily combines with carbon monoxide, hampering the desired reactions. For instance, this reaction can be seen in polymer electrolyte membrane (PEM) fuel cells. PEM fuel cells are highly probable candidates for small-scale and mobile power production, which are not based on combustion engines or batteries.
The team from Tufts illustrated that by scattering individual, isolated platinum atoms in economical copper surfaces, an highly effective and cost-efficient catalyst can be produced for the selective hydrogenation of 1,3 butadiene, which is a chemical generated when naphtha is steam cracked or gas oil is catalytically cracked.
Propene streams contain the impurity called butadiene, which has to be eliminated from the streams using the process of hydrogenation. This allows downstream polymer production. Silver and palladium are the two industrial catalysts currently in use for butadiene hydrogenation.
Like Sugar in Coffee
Charles Sykes, Professor of Chemistry, Ph.D., and one of the senior authors on the paper, stated that although copper is comparatively lesser in cost, it is not practically as catalytically powerful as platinum.
"We wanted to find a way to improve its performance."
At first, the Tufts researchers performed surface science analyses to accurately understand the mixing mechanism of platinum and copper.
"We were excited to find that the platinum metal dissolved in copper, just like sugar in hot coffee, all the way down to single atoms. We call such materials single atom alloys," said Sykes.
For this purpose, a specialized low temperature scanning tunneling microscope was used by the team to view the single platinum atoms and observe how they interacted with hydrogen.
"We found that even at temperatures as low as minus 300 degrees F these platinum atoms were capable of splitting hydrogen molecules into atoms, indicating that the platinum atoms would be very good at activating hydrogen for a chemical reaction," Sykes said.
With this information in hand, the team collaborated with long-term associates Maria Flytzani-Stephanopoulos, Ph.D., the Robert and Marcy Haber Endowed Professor in Energy Sustainability at the School of Engineering, to establish what was the most favorable hydrogenation reaction for industrial applications. She provided the confirmation that it was butadiene.
Butadiene reacted as expected in laboratory vacuum conditions. Hence, Flytzani-Stephanopoulos's team decided to pursue this further. First, the researchers created small batches of catalysts, such as platinum-copper single atom alloy nanoparticles, and scattered them on an alumina substrate. Then, they exposed them under industrial temperatures and pressures.
"To our delight, these catalysts worked very well and their performance was steady for many days," said Flytzani-Stephanopoulos. "While we had previously shown that palladium would do related reactions in a closed reactor system, this work with platinum is our first demonstration of operation in a flow reactor at industrially relevant conditions. We believe this approach is also applicable to other precious metals if added as minority components in copper."
The researchers realized that when more platinum is used, the efficiency of the reaction reduced. This was because platinum atom clusters possess substandard selectivity opposed to individual atoms.
"In this case, less is more," said Flytzani-Stephanopoulos, "which is a very good thing."
Environmental Benefits
The novel economical platinum-copper catalysts are likely to help widen the acceptance of such environmentally friendly processes and devices, as platinum is used a lot in green chemicals production and clean energy technologies, such as catalytic converters, fuel cells, and value-added chemicals produced from bio-renewable feedstocks.
This discovery was possible due to the prolonged cross-disciplinary collaboration between Sykes and Flytzani-Stephanopoulos.
"Maria and I met more than seven years ago and talked regularly about how to combine our fairly different fields of research into an effective collaboration across the schools of Arts and Sciences and Engineering," said Sykes. "I had a state-of-the-art microscope that could see and manipulate atoms and molecules, and I wanted to use its unique capabilities to gain insight into industrially important chemical reactions. In the early 2000s, Maria’s group had pioneered the single-atom approach for metals anchored on oxide supports as the exclusive active sites for the water-gas shift reaction to upgrade hydrogen streams for fuel cell use. Catalyst design know-how already existed in her lab. In retrospect, it seems obvious that combining forces would be a 'natural' development. Together we embarked on a new direction involving single atom alloys as catalysts for selective hydrogenation reactions. Our microscope was uniquely suited for characterizing the atomic composition of surfaces. We got funding from the National Science Foundation, U.S. Department of Energy and the Tufts Collaborates initiative to pursue this new area of research."
Sykes and Flytzani-Stephanopoulos made use of this method to design many single atom alloy catalysts, which have captured the interest of a global audience in the recent past.
"Traditionally catalyst development happens by trial and error and screening many materials," said Flytzani-Stephanopoulos. "In this study we took a fundamental approach to understanding the atomic scale structure and properties of single atom alloy surfaces and then applied this knowledge to develop a working catalyst. Armed with this knowledge, we are now ready to compare the stability of these single atom alloy catalysts to single atom catalysts supported on various oxide or carbon surfaces. This may give us very useful criteria for industrial catalyst design."
Initially, the research was conducted by co-authors Felicia R. Lucci and Jilei Liu, senior graduate students in the Sykes and Stephanopoulos labs, respectively.
Other authors of the paper are doctoral students Matthew D. Marcinkowski (chemistry) and Ming Yang (chemical engineering), and Lawrence F. Allard, Ph.D., of the Materials Science & Technology Division, Oak Ridge National Laboratory, who headed the state of the art imaging of the catalytic samples.
The National Science Foundation grant (CBET-1159882) and the Department of Energy grant (DE-FG02-05ER15730) funded this work.
The Tufts research findings were published under the title "Selective hydrogenation of butadiene on platinum copper alloys at the single atom limit," in the Nature Communications journal (DOI: 10.1038/NCOMMS9550).