Dec 11 2015
The power of electron guns has been increased by a factor of 13,000 by the addition of tiny diamonds on the gun tip. This increase in power was also associated with an increase in accuracy. It is hoped this technique will be integarted into the next generation of particle accelerators and electron microscopes.
Fineart1 | Shutterstock
Electron guns play an important part in research and industry. They emit the streams of electrons that are required for particle accelerators, semiconductor patterning equipment and electron microscopes.
Researchers from Stanford University have developed a new method that boosts the power of electron guns whilst also giving the electron beams atomic scale accuracy.
The smallest possible pieces of diamond are named diamondoids, with each bit weighing less than a billionth of a billionth of a carat. Diamondoids have an extremely high chemical purity and are also strong and firm due to their cage-like atomic strcuture. These characteristics mean diamondoids have useful properties that diamonds of larger sizes do not have.
Researchers at Stanford had earlier, in 2007, demonstrated that a single diamondoid layer on a metal surface has the ability to emit electrons and focus them into a minute beam with a narrow energy frequency range.
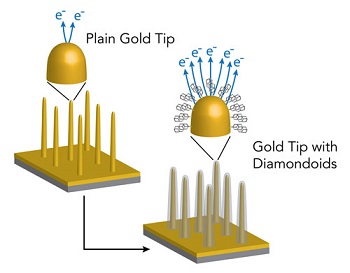
In the current study, the team attempted to find out if emissions from electron guns could be improved using a diamondoid coating. Melosh stated that making the tip very sharp could boost an electron gun's power; however, such tips are not stable.
The performance of electron guns is highly dependent on the tip remaining the right shape and their effectiveness deteriorates with even minor irregularities. Chemical coating the tips could increase electron emission, however, exposure to air makes them burn.
In this study, tiny germanium wire nanopillars were used instead of electron gun tips. The wires were initially coated with gold and then with diamondoids. Tips coated with four cage diamondoids proved to be the best as they were could release 13,000 times more electrons than a bare gold tip.
Additional tests showed that some of the diamondoid molecules were ionised due to the loss of an electron, the reason for this has not yet been established. It is this loss of electrons, but on a mass scale, that results in the manifold increase in electron release. The ionisation of the diamondoid results in a positive charge on the tip end, which draws electrons from below the surface allowing them to flow more rapidly.
Most other molecules would not be stable if you removed an electron; they’d fall apart. But the cage-like nature of the diamondoid makes it unusually stable, and that’s why this process works. Now that we understand what’s going on, we may be able to use that knowledge to engineer other materials that are really good at emitting electrons.
Nick Melosh - Stanford University