Jan 22 2016
A long-standing controversy of fuel cell catalysts has recently been solved by a research team from the Faculty of Pure and Applied Sciences, University of Tsukuba.
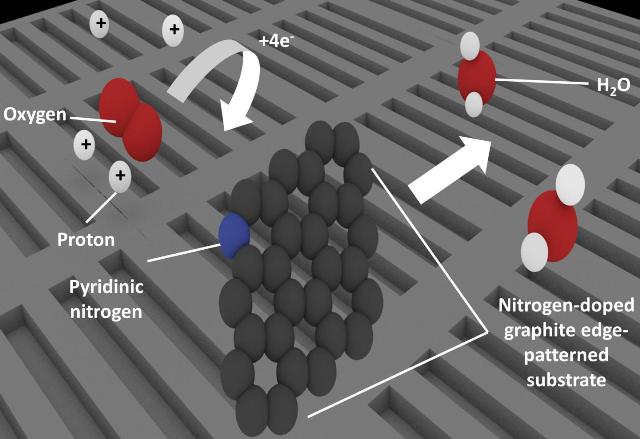 Patterning nitrogen-doped graphite to create many edges also increases the amount of pyridinic nitrogen present. Carbon atoms adjacent to pyridinic nitrogen behave as the active site for oxygen reduction, which is a key process in fuel cell technologies. (Credit: University of Tsukuba)
Patterning nitrogen-doped graphite to create many edges also increases the amount of pyridinic nitrogen present. Carbon atoms adjacent to pyridinic nitrogen behave as the active site for oxygen reduction, which is a key process in fuel cell technologies. (Credit: University of Tsukuba)
In fuel cell operation, one key steps is the oxygen reduction reaction, but the reaction depends on costly precious metal-based catalysts. Carbon-based and nitrogen-added catalysts are potential alternatives, and could allow widespread application of fuel cell technology. Until recently the arrangement of carbon and nitrogen that give the catalytic effect remained a mystery, stalling efforts in the development of more effective materials.
In an addition of the journal, Science, a research team from the University of Tsukuba have determined the catalytic structure, and put forward a mechanism to explain the working of the reaction.
We knew that nitrogen-doped carbon was a good oxygen reduction catalyst, but no one was sure whether the nitrogen was pyridinic or graphitic.
Professor Junji Nakamura, Corresponding Author
To solve the mystery, the team constructed four models of catalyst substrates. The models simulated completing potential structures, and examined their reaction performance. Pyridinic nitrogen, or nitrogen atoms attached to two carbon atoms, exists mainly at the material’s edges. By forming the substrates for changing the number quantity of edges, the research team controlled the presence of pyridinic nitrogen, and were able to measure the affected catalytic performance. These results demonstrated that pyridinic nitrogen was associated with active catalytic sites.
The team proposed the different stages of the mechanism after determining that the carbon atom adjacent to the nitrogen was the active site instead of the nitrogen atom itself.
Clarifying the active site and mechanism is a great step forward and will allow optimization studies to focus on driving up catalyst performance.
Professor Junji Nakamura, Corresponding Author