Feb 15 2016
The Okinawa Institute of Science and Technology Graduate University (OIST) aims to promote collaboration between a wide range of disciplines.
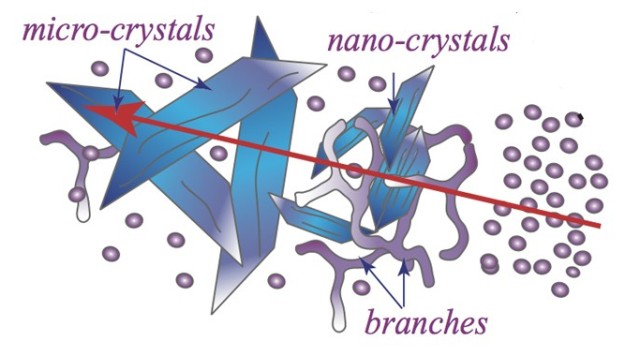
With this in focus, OIST scientists recently integrated methods from structural biology to soft matter physics to develop and visualize crystals created from surfactants, which are also known as surface-acting agents. Surfactants are used in a number of products such as paints, cosmetics, and detergents.
Crystals are high ordered arrangements of atoms in 3D. The disciplines of biology and physics are continuously searching for applications of crystals, even though the production of crystals is often a very challenging process. This interdisciplinary work, published in the Scientific Reports journal, holds potential for rapid drug discovery and delivery. Biotechnology, material sciences, and the pharmaceutical industry are other fields of applications of this breakthrough.
The scientists used a mixture of two biocompatible surfactants, Monolaurin and Tween80. Monolaurin is present in coconut oil, and Tween80 is a not-charged biocompatible surfactant mostly used in cosmetics and pharmaceutical formulations. Part of the surfactant molecule likes water and another part repels it. This is the reason that surfactants form micelles when they are dissolved in water. Micelles are spherically shaped aggregates containing the water-friendly chemical groups arranged on the outside. The transformation of spherical micelles to crystals is a complicated procedure involving a number of shape changes.
OIST scientists discovered that under the correct temperature, the micelles confined in a narrow space along with shear flow, being organized into varied shapes.
After just 5 minutes, we saw that the samples became turbid and milky. We asked our colleagues of the Structural Cellular Biology unit to have a look at them and we found out that the milky solution contained worm-like micelles, branched micelles and crystal-like structures.
Dr. Joshua Cardiel, PhD. Student
OIST’s Structural Cellular Biology unit, headed by Prof. Ulf Skoglund, used cryogenic electron-microscopy (cryo-EM), which is where the sample is frozen and then examined under temperatures close to -200°C. Cryo-EM photos highlight that the sample appears to be cloudy because of the transformation from spherical micelles into well arranged structures. The spherical micelles are arranged into wormlike micelles, and then form branched micelles. This is followed by the final transition into ordered crystal-like structures.
Traditional crystallography is based on removing water from the sample chemically, so called 'salting out', usually associated with smaller temperature variations to spot optimal crystallization conditions. Our method took advantage of adding external shear flow, combined with ambient temperatures to induce controlled crystallization.
Professor Amy Shen, Postdoctoral Researcher, National Institute of Standards and Technology
“Every crystallographer knows that obtaining a good crystal is a very long and challenging process. This technique has the potential to reproducibly create crystals that are only 0.1 µm, which is 100 times smaller than the current ones used in X-ray crystallographic studies,” comments Prof. Skoglund. “We will continue to work together with physicists to explore the possibilities of this new method.”