Feb 24 2016
Lithium-ion batteries gained popularity in the 1990s, and have lead to the replacement of nickel-metal hydride batteries. Today Li-ion batteries power numerous electronic gadgets, such as mobile, laptops, and tablets.
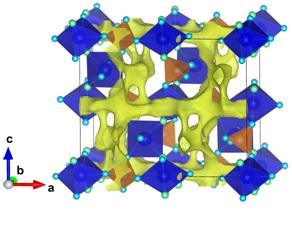 Polyhedral representation of the crystal structure of fluoride-phosphate of vanadium and potassium. The yellow denotes a three-dimensional channel system, which provides rapid transport of Li+ ions. (Photo Credit: Stanislav Fedotov)
Polyhedral representation of the crystal structure of fluoride-phosphate of vanadium and potassium. The yellow denotes a three-dimensional channel system, which provides rapid transport of Li+ ions. (Photo Credit: Stanislav Fedotov)
These batteries do have certain disadvantages, for instance when the temperature drops below zero their capacity may decline. Another downside is the price due to the use of expensive lithium-containing materials. The cost of Li-ion batteries represents half of the price of a renowned electro car Tesla Model S.
Positive features of the Li-ion batteries include ease of use, high volume, and compactness, which means that the gadget would enjoy an extended service life despite the relatively small battery.
The Li-ion battery’s capacity is hindered by the material used to make its cathode. Researchers have already studied a vast number of the existing materials, and continue to search for new cathode materials with the capacity to recharge fully in a matter of minutes, function under high current densities, and store additional energy.
Fluoride-phosphates of transition metals have been identified as one of the most probable group of cathode materials for advanced Li-ion batteries.
Prof. Evgeny Antipov, correspondent member of the Russian Academy of Sciences and the head of the MSU Electrochemistry Department, headed a study involving scientists from MSU, and Russian and Belgian scientists. The research team created an innovative high-power cathode material for Li-ion batteries using a fluoride-phosphate of potassium and vanadium.
The work is based on a simple idea of geometric and crystal-chemical conformity of ionic sublattices.
Stanislav Fedotov, Junior Research Scientist, Electrochemistry Department, Faculty of Chemistry, MSU
The team successfully stabilized an innovative crystal structure, which provides fast transportation of lithium ions via spatial channels and cavities. The recommended cathode material displayed high charge/discharge rates measuring down to 90 seconds. As a result the material was able to retain over 75% of the original specific capacity.
Once the composition and morphology is tweaked, the novel material is likely to become a major competitor to popular and commercialized high-power cathode materials, such as NaSICON.
The team believes that its research could create new opportunities for searching and the synthesis of innovative cathode materials for Li-ion batteries, as well as advance the progress of a unique battery type, where the role of a mobile ion would be carried out by potassium ions rather than by lithium.
It is assumed that such batteries would not only deliver high energy density, but would also be economically attractive due to a replacement of expensive lithium-containing components with cheaper and hence affordable potassium-containing analogue.
Stanislav Fedotov, Junior Research Scientist, Electrochemistry Department, Faculty of Chemistry, MSU
The research findings have been published in Chemistry of Materials (current IF -- 8.354).