Mar 16 2016
In order to study the effects of microgravity in a better way, Japanese researchers have grown protein crystals and successfully determined the rate of the crystals’ growth onboard the International Space Station (ISS) using laser interferometry.
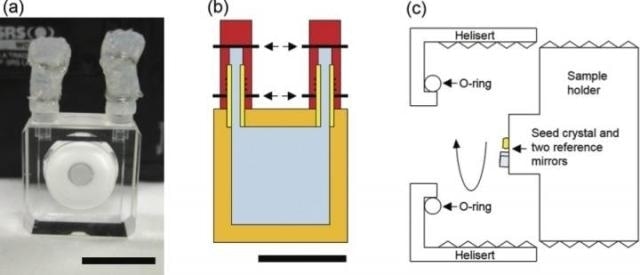 (a) The white ring is a ceramic helical insert on which the screw sample holder is placed. The scale bar is 10 mm. (b) The body of the growth cell is made of quartz glass (orange). Two capillaries of quartz glass with rubber stoppers (yellow) are fixed to the body with an adhesive. Tubes of elastomer (red) are attached to each capillary. After the growth cell is filled with the growth solution (light blue), the tubes are closed with metal wires (arrows). (c) Schematic illustration of the growth cell. (CREDIT K. Tsukamoto et al/Tohoku University).
(a) The white ring is a ceramic helical insert on which the screw sample holder is placed. The scale bar is 10 mm. (b) The body of the growth cell is made of quartz glass (orange). Two capillaries of quartz glass with rubber stoppers (yellow) are fixed to the body with an adhesive. Tubes of elastomer (red) are attached to each capillary. After the growth cell is filled with the growth solution (light blue), the tubes are closed with metal wires (arrows). (c) Schematic illustration of the growth cell. (CREDIT K. Tsukamoto et al/Tohoku University).
Distance can sometimes provide a new outlook to an issue. For the research team analyzing the growth of protein crystals, that distance was 250 miles up – the altitude at which the planet is orbited by the ISS.
Katsuo Tsukamoto's research group, from Tohoku University's Department of Earth and Planetary Science in Sendai, Japan, and the Japan Aerospace Exploration Agency, grew crystals in a specially-designed chamber on the ISS. This was done to isolate the protein crystal growth from the effects of gravity.
Using laser interferometry the team tracked the slow growth and the dissolution speed of the crystals. This rate was roughly 1 cm/second of the crystals. It was the first time that this method had been implemented onboard the ISS to determine the rate of crystal growth at different temperatures. To visualize this method the researchers developed unique growth cells, which are perfect for long-standing projects of roughly six months.
We are interested in the growth mechanisms of a space-grown protein crystal -- a lysozyme crystal -- as a model crystal to understand why space-grown crystals sometimes do show better quality than the Earth-grown crystals.
Tomoya Yamazaki, Ph.D student, Tohko University
Tsukamoto and his team, which comprises collaborators at Japan Space Forum, the Japan Aerospace Exploration Agency, Olympus Optical, have described their well-organized growth technique in AIP Publishing’s Review of Scientific Instruments.
Called NanoStep, the experimental method was carried out in the Japanese Experimental Module (KIBO) of the ISS back in 2012. The rate of protein crystal growth had already been measured by the research team under replicated microgravity, using a Russian recoverable aircraft and satellite in parabolic flights. The group took accurate measurements of the rate of lysozyme crystal growth against their supersaturation - the natural logarithm of the concentration of the protein divided by its solubility - with measurements of the refractive index distribution of a solution acquired through interferometry. This also provided important data regarding the growth mechanism.
The team decided to modify the solutions supersaturation by either decreasing or increasing the temperature of the growth cell. This occurred over a range of 10 to 40oC and can also be performed remotely. As a result, a closed, cube-like growth cell was constructed to endure the stresses caused by the solution’s thermal expansion. The growth cell was developed from quartz glasses with varied thickness, which is a major component for interferometry, due to its high mechanical and chemical resistance, with a protein seed crystal fixed to the top of the screw sample holder. In order to ease the glass’ thermal stress, the research team fixed elastomer-based tubes. Elastomer is a thermoelastic polymer with low-moisture-permeability. This polymer was used to reduce water evaporation in the crystal growth solution. The solution included 25 mg/ml sodium chloride and 30 or 35 mg/ml of lysozyme in 50 mM sodium acetate buffer solution. A unique spring tension system was also used to reduce stress, by ensuring that the gap between the thermal control modules and the glass cell remains constant between thermal expansions.
With the aid of the growth cell, the measurements of small dissolution and growth rates of insoluble minerals can be adjusted on the order of 0.001 nm/s. For instance, the growth cell is capable of determining crystals of calcium carbonate, in which the error margins could turn out to be substantial over a geological time scale, such as predicting the dissolution rate of clay minerals covering the nuclear waste kept within the ground for 100,000 years.
The researchers anticipated a slower growth rate with regard to the crystal solution, due to the suppression of solution convection, but instead the results revealed an increased growth rate. This could be attributed to the suppression of transport rate of impure, larger diameter molecules towards the growing crystal, as studied in the supersaturation relations vs. growth rate. This work will be published in upcoming papers.
Preparations are being made for extended projects adopting the same apparatus to study the growth of glucose isomerase crystals and other crystals.