Apr 22 2016
A high-precision method of producing electrodes, developed by a group of researchers, would enable hybrid batteries that charge quicker than traditional ones and have a much better electrical capacity and extended stability.
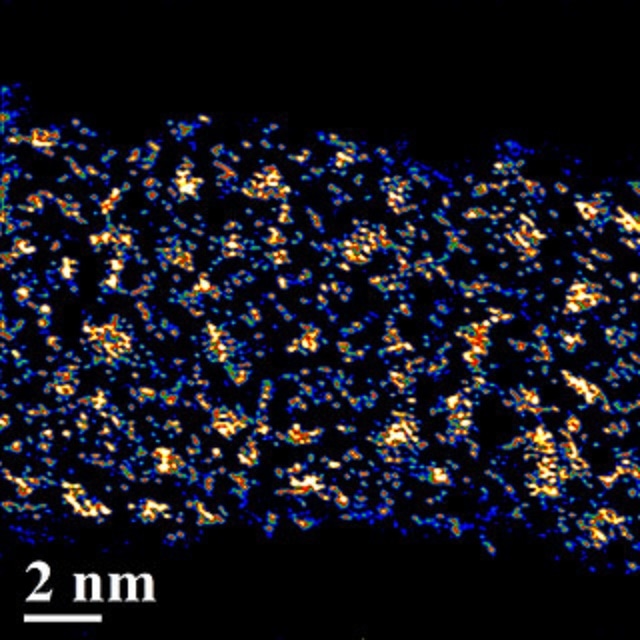 Ion soft landing distributes negative POM ions (bright spots) evenly onto a supercapacitor, leaving unwanted positive ions behind. (Photo credit: Venkateshkumar Prabhakaran/PNNL)
Ion soft landing distributes negative POM ions (bright spots) evenly onto a supercapacitor, leaving unwanted positive ions behind. (Photo credit: Venkateshkumar Prabhakaran/PNNL)
This novel method, dubbed ion soft-landing, has made it possible for electrodes to store a third more energy and have twice the service life compared to electrodes prepared using the
traditional technique.
The research findings have been reported in Nature Communications.
The novel technique is uncomplicated to set up and it could gradually lead to more economical, powerful rechargeable batteries with extended life.
This is the first time anyone has been able to put together a functioning battery using ion soft-landing.
Julia Laskin, Chemist and Laboratory Fellow, Department of Energy, Pacific Northwest National Laboratory
The benefits are possible due to soft-landing's ability to construct an electrode surface very distinctively, using only highly preferred molecules out of a complicated mixture of raw components.
It will help us unravel important scientific questions about this energy storage technology, a hybrid between common lithium rechargeable batteries and supercapacitors that have very high energy density.
Venkateshkumar Prabhakaran, Chemist, PNNL
A different kind of hybrid
Most small electronic devices are powered by lithium ion rechargeable batteries; however, they discharge their energy slowly. This is why hybrid electric vehicles use gasoline to accelerate, and the charging time is longer. As a result, electric vehicles take more time to fill, compared to their gas-powered counterparts.
One potential solution is a hybrid battery, which has lithium battery's ability to contain a large amount of charge for its size and a quick-charging supercapacitor.
PNNL chemists were keen to know if they could make better hybrid battery materials using ion soft-landing, which carefully controls the raw parts during preparation.
As part of the effort, Laskin and his colleagues developed hybrid electrodes by spraying a chemical known as polyoxometalate (POM) onto supercapacitor electrodes composed of microscopically small carbon tubes.
Commercial POM possesses positively and negatively charged parts, known as ions. Conversely, hybrid electrodes require only the negative ions. The traditional preparation method is restricted by its design, spraying both the negative as well as the positive ions onto the carbon nanotubes.
However, ion soft-landing divides the charged components and only the negative ions are placed on the electrode surface. The key query Laskin and his team had related to the positive ions’ interference with the performance of hybrid electrodes. To understand further, the team built centimeter-sized square hybrid batteries, made up of POM-carbon nanotube electrodes that sandwiched a specifically created ionic liquid membrane between them.
We had to design a membrane that separated the electrodes and also served as the battery's electrolyte, which allows conduction of ions. Most people know electrolytes as the liquid sloshing around within a car battery. Ours was a solid gel.
Venkateshkumar Prabhakaran, Chemist, PNNL
They analyzed a mini-hybrid battery to test the amount of energy it can contain, and the number of cycles of charging and discharging it could handle before failing. They compared soft-landing with traditionally created hybrid batteries, which were constructed using a technique known as electrospray deposition. They used a commercial POM, containing positively charged sodium ions.
Cheers for the POMs
When soft-landing was used, the researchers discovered that the POM hybrid electrodes possessed excellent storage capacity. They contained a third more energy compared to the carbon nanotube supercapacitors on their own, which were added as a minimum performance standard. Soft-landing hybrids contained nearly 27% additional energy than traditionally manufactured electrospray deposited electrodes.
To ensure the optimal quantity of POM was being utilized, they created hybrid electrodes using various quantities and analyzed which one resulted in the maximum capacity. Soft-landing generated the highest capacity overall, using the lowest quantity of POM. This indicated that the electrodes used the active material very efficiently. In comparison, traditional, sodium-based POM electrodes needed twice as much POM material to achieve their maximum capacity.
The traditionally created devices used additional POM, but the researchers are not able to quantify the amount yet. It is possible that they possess a longer service life than the soft-landing produced electrodes. The researchers validated the length of the service life of hybrids by charging and discharging them 1,000 times.
As they did in the earlier evaluations, the soft-landing-based gadgets performed excellently, losing only a few percent of capacity after 1000 cycles. The naked supercapacitors came second, while the sodium-based, traditionally manufactured devices lost nearly twice the capacity of the soft-landed devices. This indicates that the soft-landing technique has the potential to increase the lifespan to twice the amount of these types of hybrid batteries.
Looking good
Only a limited amount of POM material was used to create such a huge impact on the carbon nanotube supercapacitors. Just one-fifth of a percent of the quantity of carbon nanotube material was used.
The fact that the capacitance reaches a maximum with so little POM, and then drops off with more, is remarkable. We didn't expect such a small amount of POM to be making such a large contribution to the capacitance.
Julia Laskin, Chemist and Laboratory Fellow, Department of Energy, Pacific Northwest National Laboratory
They decided to inspect the structure of the electrodes with the aid of powerful microscopes in the Environmental Molecular Sciences Laboratory (EMSL), a DOE Office of Science User Facility at PNNL. They analyzed soft-landing with the traditionally manufactured sodium-POM electrodes.
Soft-landing developed small distinct clusters of POM dotting the carbon nanotubes, but the outcome of the traditional method was larger clumps of POM clusters crowding the nanotubes, with aggregates in the sizes of up to ten times that of those produced by soft-landing.
This outcome meant that eliminating the positive ions from the POM starting material allowed the negative ions to spread uniformly over the surface. Until the positive ions like sodium remained, the sodium and POM appear to improve the crystalline material and aggregate on the surface. This prevented a lot of the POM from performing its job in the battery, thereby decreasing capacity.
By zooming out and viewing the nanotubes from above, the team were able to see that the traditionally manufactured electrodes were coated with large POM aggregates. However, it was virtually impossible to differentiate the soft-landed electrodes from the naked carbon nanotube supercapacitors.
Going forward, the team plan to study ways to obtain the carbon materials to add more POM, which may enhance the capacity and service life much more.
The Joint Center for Energy Storage Research, a Department of Energy Innovation Hub, and the Department of Energy Office of Science supported the research work.