May 9 2016
Generally, in rechargeable batteries, lithium ions are transported by the electrolyte from the negative electrode to the positive electrode during the discharging process and the ionic flow path reverses during the recharging process.
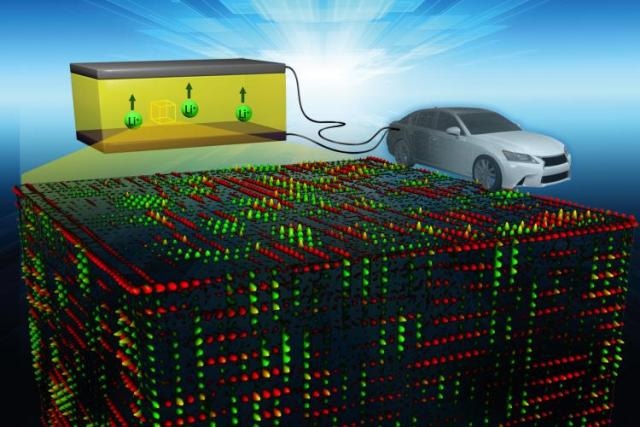 An ORNL-led research team found the key to fast ion conduction in a solid electrolyte. Tiny features maximize ion transport pathways, represented by red and green. Image credit: Oak Ridge National Laboratory, U.S. Dept. of Energy
An ORNL-led research team found the key to fast ion conduction in a solid electrolyte. Tiny features maximize ion transport pathways, represented by red and green. Image credit: Oak Ridge National Laboratory, U.S. Dept. of Energy
In commercially available lithium-ion batteries, the organic liquid electrolytes are flammable and are often subject to leakage. As a result, large-scale application of organic liquid electrolytes is a challenging prospect. In contrast solid electrolytes are capable of resolving these problems; however, their ionic conductivity is characteristically low.
A research team, headed by the Department of Energy’s Oak Ridge National Laboratory (ORNL) have applied an advanced microscopy technique to detect an unknown feature, measuring about 5 nm or billionths of a meter wide, in a solid electrolyte. At an experimental level, the study validates the feature’s significance to rapid ion transport, and substantiates the findings with theory. The novel mechanism, reported by the researchers in Advanced Energy Materials, describes a new approach for designing highly conductive solid electrolytes.
The solid electrolyte is one of the most important factors in enabling safe, high-power, high-energy, solid-state batteries, but currently the low conductivity has limited its applications.
Cheng Ma, Postdoctoral Associate, ORNL
ORNL’s Miaofang Chi, the senior author, said, “Our work is basic science focused on how we can facilitate ion transport in solids. It is important to the design of fast ion conductors, not only for batteries, but also for other energy devices.” These comprise pf fuel cells and supercapacitors.
In order to directly view the arrangement of atoms in the solid electrolyte, the team utilized aberration-corrected scanning transmission electron microscopy to transmit electrons via a sample. To view a very small feature in a 3D material with a technique that inherently gives a 2D projection, a sample of unusual thinness was required. To formulate such a sample, the team leveraged the all-inclusive materials processing and characterization capabilities of the Center for Nanophase Materials Sciences - a DOE Office of Science User Facility at ORNL.
Usually the transmission electron microscopy specimen is 20 nanometers thick, but Ma developed a method to make the specimen ultra-thin (approximately 5 nanometers). That was the key because such a thickness is comparable to the size of the hidden feature we finally resolved.
Miaofang Chi, Research Staff, ORNL
The team then went on to investigate a prototype system known as LLTO, which stands for lithium, lanthanum, titanium and oxygen building blocks. Among oxide systems, LLTO is known to have the highest bulk conductivity. It was observed that lithium ions in this material move at the fastest speed in the planar 2D pathways, resulting from irregular stacks of atomic layers rich in lithium or lanthanum.
The researchers observed that, without damaging this enhanced 2D transport, fine features or small domains measuring 5 to 10 nm wide across the 3D material, provided increased number of directions in which lithium ions can move. The domains resembled sets of shelves stacked at right angles to others. When the shelves are smaller, it was easier for the ions to flow towards the direction of an applied electrical energy.
Yongqiang Cheng and Bobby Sumpter from ORNL carried out molecular dynamics simulations, which substantiated the experimental results. Earlier, scientists tend to look at the atomic structure of the most basic repeating unit of a crystal known as unit cell, and they either reorganize its atoms or add varied elements to observe how they could enable ion transport.
Unit cells usually measure less than 1 nm wide. In the material studied by ORNL team, the unit cell measured about half a nanometer. The researchers eventually gained a new perspective that fine features, measuring just a few nanometers and crossing several unit cells, can actually increase the number of ionic transport pathways.
The finding adds a new criterion. This largely overlooked length scale could be the key to fast ionic conduction.
Miaofang Chi, Research Staff, ORNL
When designing materials for rapid ion conduction, scientists would need to account for phenomena on the order of several nanometers.
Ma agreed. “The prototype material has high ionic conductivity because not only does it maintain unit-cell structure, but also it adds this fine feature, which underpins 3D pathways,” Ma said. “We’re not saying that we shouldn’t be looking at the unit-cell scale. We’re saying that in addition to the unit cell scale, we should also be looking at the scale of several unit cells. Sometimes that outweighs the importance of one unit cell.”
For many years, when there was no explanation for specific behaviors of materials, researchers contemplated that phenomena exceeding a single unit cell may be responsible; however, no such proof was available on this.
This is the first time we proved it experimentally. This is a direct observation, so it is the most solid evidence.
Cheng Ma, Postdoctoral Associate, ORNL
The paper is titled “Mesoscopic Framework Enables Facile Ionic Transport in Solid Electrolytes for Li-ion Batteries.”
The theory calculations, electron microscopy, and electrochemical analysis were supported by the DOE Office of Science. The study was carried out at the Center for Nanophase Materials Sciences, a DOE Office of Science User Facility at ORNL. Scientists at Tsinghua University in China took part in electrochemical analysis and materials synthesis.