Jul 7 2016
A new catalyst that can simplify the process of synthesis of substituted 1H-indenes has been created by a group of scientists in Sustainable Chemistry, a predominant research area in the University of Amsterdam. The catalytic complex of the earth-abundant cobalt is inexpensive and can be developed in a simple way. This catalytic complex is capable of the sustainable concept of metalloradical catalysis. Information related to this research has been presented in the website of the Journal of the American Chemical Society.
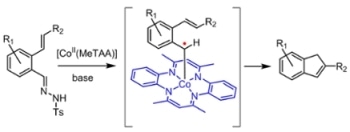 Thermolysis of the applied tosyl hydrazones (easily prepared by condensation of the corresponding aldehydes with tosyl hydrazine) leads to in situ formation of the corresponding diazo compounds. The latter are activated by the catalyst, thus forming the key carbene-radical intermediates responsible for radical-type ring-closure to form the final indene products. The reaction has a broad substrate scope, tolerant to various different substituents, enabling facile synthesis of a variety of indene derivatives. (Credit: HIMS / Homkat.)
Thermolysis of the applied tosyl hydrazones (easily prepared by condensation of the corresponding aldehydes with tosyl hydrazine) leads to in situ formation of the corresponding diazo compounds. The latter are activated by the catalyst, thus forming the key carbene-radical intermediates responsible for radical-type ring-closure to form the final indene products. The reaction has a broad substrate scope, tolerant to various different substituents, enabling facile synthesis of a variety of indene derivatives. (Credit: HIMS / Homkat.)
Many bio-active compounds, natural products and pharmaceuticals have indenes as a significant part of their structure. Indenes also have numerous uses for catalytic purposes such as olefin polymerization, in metal complexes. There is a high demand for quicker and more efficient means of indene synthesis that can be applied for various purposes and can be developed using easily available raw materials.
Bio-Inspired Approach
Scientists from UvA’s SusChem group have developed a new strategy that can contribute to the development of such new methods. This strategy offers a sustainable catalytic route for the synthesis of indene. The team’s new, bio-inspired perspective in the area of metallo-radical catalysis has been the root-cause to this development. Unlike the commonly used catalytic methods that prevent radical formation, the bio-inspired approach exploits the radical-type reactivity in first row transition metals.
The notion of metallo-radical catalysis allows scientists to use inexpensive metals in place of high-priced noble metal catalysts that are commonly used, notes research head Bas de Bruin. Drawing inspiration from the operation of natural metallo-enzymes, De Bruin and team are currently using the notion of radical reactivity to develop more catalysts in the future.