Jul 7 2016
Chiral molecules are asymmetric in nature, like left and right hands, so superposing their mirror images is not possible. Molecules with matching chemical structure but different chirality can exhibit different activity. This makes chirality is a crucial factor in fields such as chemistry, biology, and pharmacology. Synthesis of molecules with specific chirality can be tricky, however it is a key goal of several chemists as they search for desired materials.
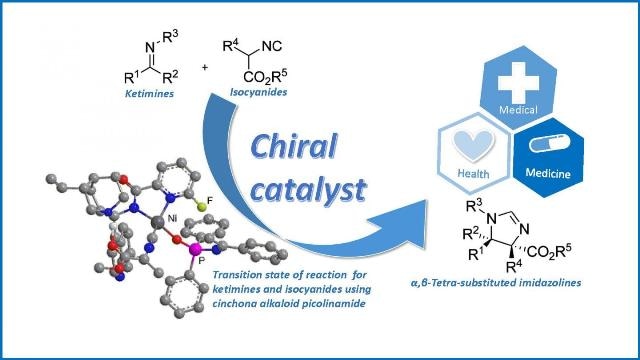 Authors successfully synthesized high yields of chiral 2-imidazolines (alpha,beta-Tetra-substituted imidazolines) from Ketimins and isocyanides via direct catalytic Mannich reaction. This approach can be a clue to develop novel and effective pharmaceutical products. (Photo Credit: NITech)
Authors successfully synthesized high yields of chiral 2-imidazolines (alpha,beta-Tetra-substituted imidazolines) from Ketimins and isocyanides via direct catalytic Mannich reaction. This approach can be a clue to develop novel and effective pharmaceutical products. (Photo Credit: NITech)
Researchers at the Nagoya Institute of Technology have recently synthesized various types of chiral molecules using the catalytic Mannich reaction. They published their findings in the Royal Society of Chemistry's ChemComm. The novel synthesized chiral molecules are made up of imidazoline rings, which are five-membered rings containing three carbon and two nitrogen atoms. Materials with these rings could be useful as pharmaceutical agents and catalysts.
The team discovered that the catalytic Mannich reaction is an effective technique to synthesize chiral imidazolines from ketimines and α-isocyanoacetates. This is a vital finding as ketimines are commonly considered poor base materials due to their low reactivity and weak ability to develop preferred chiral molecules.
In their synthetic method, the team first established the optimal conditions to accomplish high yields of imidazolines with definite types of chirality.
Performing the Mannich reaction with a certain cinchona alkaloid catalyst gave a product with high stereoselectivity, so molecules with a desired specific configuration were generated. Using a different catalyst gave the corresponding product with the opposite chirality.
Shuichi Nakamura, Lead Author
Through analysis of the optimal reaction conditions, the researchers then conducted the catalytic Mannich reaction using a range of different ketimines and isocyanoacetates. They discovered that the reaction was successful in relation to several of these materials to offer chiral products with stereoselectivity and high yield. Subsequently, the products were converted into chiral imidazolines by eliminating a protecting group.
Until now, no method had successfully synthesized vicinal tetrasubstituted imidazolines effectively and efficiently. This is the first example of highly stereoselective synthesis of imidazolines through reaction of non-activated ketimines with isocyanides.
Shuichi Nakamura, Lead Author
The outcome of the research reveals a new path to acquire complex, versatile materials from base materials with low reactivity.