Distek, Inc., a leading manufacturer of laboratory testing instruments for the pharmaceutical and biotechnology industry, as well as an experienced provider of validation and qualification services, announced today the release of the Opt-Diss 410 next generation in-situ UV fiber optic system for dissolution testing.
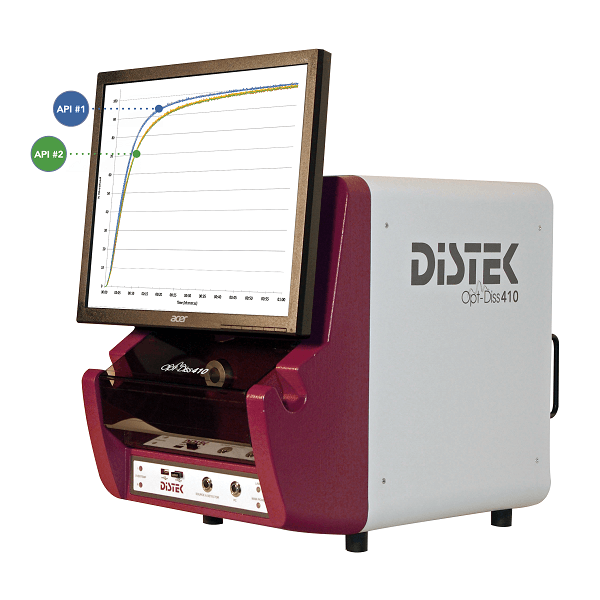
No more sampling! The Distek Opt-Diss 410 UV fiber optic system for dissolution testing measures directly in the vessel, eliminating problematic filters, tubing and pumps used in conventional sampling. Patented ARCH probes designed specifically for dissolution eliminate bubbles and trapped particles that plague other fiber systems. New multicomponent analysis allows quantifying two components at once, without the need for separation. The Opt-Diss 410 is the only fully integrated UV fiber optic dissolution solution, with a single software package controlling the entire system.
The addition of multi-component analysis is a tremendous leap forward for Distek, fiber optic dissolution and our customers. In today’s high pressure environment to produce more with less the added ability of the Opt-Diss 410 to perform multi-component analysis allows our customers to significantly reduce the turnaround time of results for an expanded number of dissolution methods by eliminating the need for LC while simultaneously reducing the costs of those results.
Jeff Seely, Distek’s Vice President of Sales & Business Development.
To learn more about the Opt-Diss 410, contact Distek’s Corporate Office at +1 732 422 7585, by email at [email protected] or visit www.distekinc.com.
Distek, Inc., headquartered in North Brunswick, NJ, offers a robust product portfolio including water bath and bathless dissolution, dissolution media heating, degassing, dispensing and disposal, in-situ fiber optic UV, bathless tablet disintegration, content uniformity, digital video monitoring, programmable automated sampling and now a single-use bioreactor system for mammalian cell culture applications.